GEXVal and FRAXA collaborate to advance Fragile X research with the Phase 2a trial of GXV-001, supported by AMED’s funding program.
Read moreFRAXA-DVI
BK Channel Openers: A New Hope for Fragile X Treatment – Insights from Kaerus Bioscience CEO Robert Ring

Kaerus Bioscience’s BK channel openers for Fragile X syndrome are advancing through Phase 1 trials, offering hope for new treatments with FRAXA’s continued support.
Read moreMarvel Biosciences Partners with FRAXA to Test MB204 for Fragile X Syndrome

Discover how Marvel Biosciences and FRAXA Research Foundation are collaborating to test MB204, a promising new treatment for Fragile X syndrome, building on groundbreaking adenosine receptor research.
Read moreFragile X Treatment Target Emerges from Neurolixis & FRAXA Collaboration
A new treatment target for Fragile X syndrome has emerged from multiple research labs and a pharmaceutical startup. Neurolixis, a FRAXA pharma partner, has announced a new Fragile X development program.
Read moreBK Channel Openers: A New Drug for Fragile X Is Ready for Clinical Trials
Discover the promising new BK channel opener, SPG601, now entering clinical trials for Fragile X syndrome. Learn about its potential to restore synaptic function and address core symptoms.
Read moreInside the FRAXA Drug Validation Initiative: Advancing Fragile X Treatments

Explore how the FRAXA Drug Validation Initiative is revolutionizing Fragile X syndrome treatment, leading the charge towards innovative therapies and hope for affected families.
Read moreTwo-Med Combo Normalized Behavior, Improved Memory in Fragile X Mice
Treating Fragile X syndrome will likely require a combination of drugs, as a single medication may not address all symptoms. At FRAXA-DVI, Dr. Patricia Cogram and her team recently tested a combination of two investigational new drugs in Fragile X mice, with support from Healx. Together ibudilast and gaboxadol rescued a wide array of symptoms in the mice.
Read moreComing Together for Rare Disease Day 2023

Today, February 28, we mark Rare Disease Day, a day dedicated to raising awareness about rare diseases and highlighting the need for continued research and collaboration. At FRAXA Research Foundation, we are committed to advancing research on Fragile X syndrome, one of the most common rare diseases worldwide.
Read moreFragile X Clinical Trial of New PDE4D Inhibitor from Tetra

With a $200,043 grant from FRAXA Research Foundation, Dr. Elizabeth Berry-Kravis completed a successful Phase 2 clinical trial of a PDE4 inhibitor for adult men with Fragile X syndrome. This trial treated 30 males, 18-45 years of age with a new PDE4D allosteric inhibitor from Tetra Discovery Partners using a crossover design, so that everyone got active drug for part of the time and placebo for part of the time.
Read moreNew Fragile X Clinical Trial Announced by Healx

Healx’s AI-driven approach makes finding the right combination therapies more efficient, cost-effective, and rapidly ready for testing at FRAXA-DVI. It was this process that has brought Healx to its recent announcement sharing that it has received Investigational New Drug (IND) approval from the US Food and Drug Administration (FDA) for the Phase 2a clinical study of HLX-0201 (sulindac, an FDA-approved drug).
Read morePurposeful and FRAXA Partnership Leads to Clinical Trial

Can a combination of drugs make a meaningful difference for people with Fragile X? A new clinical trial is going to find out. 15-20 adult men with Fragile X will be included in this trial to test the effects of an available drug and a nutritional supplement taken together.
Read moreMaking Drug Development Efficient Through Community-Based Collaboration

FRAXA co-founders Katie Clapp and Mike Tranfaglia, spoke virtually at the 5th Pharma Pricing Reimbursement and Market Access 2021 conference.
In this session, facilitated by Nadia Bodkin, PharmD, MS, from the Rare Advocacy Movement, Katie and Mike were joined by Christopher U. Missling, PhD, President and CEO of Anavex Life Sciences Corp. to discuss the collaboration between FRAXA and Anavex as a case study example to help raise awareness amongst others in the rare disease industry of these types of collaborations between advocacy and industry.
Read moreTetra’s Fragile X Clinical Trial – The Most Successful So Far

Dr. Mark Gurney, CEO of Tetra Therapeutics, discusses how one of the earliest clues to the biology of Fragile X led to the most successful Fragile X clinical trial to date. FRAXA and Tetra began working together after a key FRAXA-funded study caught the attention of Dr. Gurney. Through the FRAXA Drug Validation Initiative, Dr. Patricia Cogram was able to conduct preclinical validation experiments with Tetra’s lead compound in record time, paving the way for clinical trials.
Read morePromising Results of Preclinical Study of ANAVEX®2-73

We are excited to share that Anavex Life Sciences announced today that preclinical data of the ANAVEX®2-73 (blarcamesine) study in Fragile X syndrome were published in the peer-reviewed journal, Scientific Reports.
Read moreSynaptogenix Announced Intention to Launch a Fragile X Clinical Trial with Bryostatin

One of the most exciting kinds of work that FRAXA does is following the journey of an experimental new treatment until it is ready for trials in people with Fragile X. From an initial idea, through the development process, to clinical trials, FRAXA helps out all along the way. From an initial idea, through the development process, to clinical trials, FRAXA helps out all along the way. The recent announcement by Synaptogenix is a great example of how FRAXA funding and use of FRAXA-DVI can accelerate research on Fragile X.
Read moreFRAXA Drug Validation Initiative (FRAXA-DVI)

The FRAXA Drug Validation Initiative (FRAXA-DVI) provides speedy, cost-effective, objective preclinical testing of potential Fragile X treatments. FRAXA-DVI uses in-vitro systems, behavior batteries, and gene expression and peripheral biomarker platforms to validate investigational new drugs and repurposed available compounds in Fragile X syndrome (FXS).
Read moreTetra Releases Full Results of FRAXA-Funded Clinical Trial of PDE4D Inhibitor

Today, Tetra Therapeutics published the full results of its PDE4D trial published the full results to their announcement. Now having reviewed the full results, FRAXA can confidently say that the PDE4D drug trial gives hope to patients and families that Fragile X Syndrome is a treatable disorder, and this particular drug can improve intellectual disability.
Read moreTrofinetide Clinical Trial Results Published
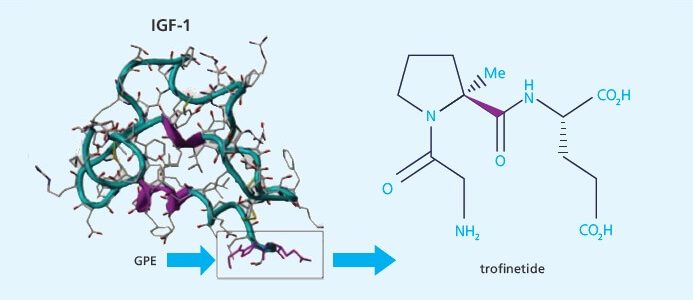
New Zealand-Based Biotech Neuren Pharmaceuticals Has Published Successful Phase 2 Fragile X Clinical Trial. Trofinetide, given to adolescent and adult males with Fragile X syndrome, was shown to be generally safe and was well-tolerated. It also showed preliminary evidence of efficacy. This trial validated a new design which can be used in future trials.
Read moreHealx Raises $56M to use AI to Find Treatments for Fragile X & Other Rare Diseases

Healx has secured $56M in new financing to build a clinical-stage portfolio for rare diseases, including treatments for Fragile X syndrome, and to launch a global Rare Treatment Accelerator program. Where the traditional drug discovery model takes more than a decade and can run into the billions of dollars, Healx’s AI-driven approach makes the process faster, more efficient and cost-effective.
Read moreTetra Announces $40M to Advance BPN14770 for FXS and Alzheimer’s Disease

Tetra Discovery Partners has signed a multi-part deal that could bring it up to $160 million, plus royalties, from Shionogi & Co, Ltd, a Japanese major research-driven pharmaceutical company. Tetra currently is conducting an investigational Phase 2 study of BPN14770 in adults with Fragile X Syndrome, an indication for which BPN14770 has received Orphan Drug Designation from the US Food and Drug Administration. This clinical trial was made possible by early work with the FRAXA-DVI and over $200,000 from FRAXA.
Read moreFinding Fragile X Biomarkers – From Transcriptomics to Behavior in Patients

With this $20,000 award from FRAXA Research Foundation, Dr. Vanderklish and collaborators at Scripps Research Institute, the University of Chile, and the FLENI Institute in Argentina are analyzing patterns in gene expression in blood cells of patients with Fragile X syndrome. They are using “transcriptomics” which can produce a time-sensitive signature of an individual person. This is the first time that all these different levels of study – from transcriptomics to behavior – have been done for individual patients with Fragile X.
Read moreFRAXA Research Grants Drive Big Investments in Fragile X

Most people know that FRAXA supports academic research at many institutions such as Harvard University, University of Pennsylvania, Massachusetts Institute of Technology, and Yale University. However, FRAXA is also working with more than 30 pharmaceutical companies around the world. Mike spends a lot of his time advising and collaborating with industry partners.
Read moreRepurposing Study II: Evaluating Combinations of Drugs to Treat Fragile X

FRAXA Research Foundation initially partnered with Healx in 2016 to identify existing drugs with potential to treat Fragile X syndrome, using machine learning algorithms and computational biology. The study produced results, and now FRAXA and Healx have launched a new round of studies to evaluate combinations of compounds, including both drugs and natural products.
Read moreDrug Repurposing Study Results Accelerate Progress Towards Fragile X Treatments

While there are over 8,000 rare diseases affecting an estimated 350 million people worldwide, only around 200 of these conditions have effective treatments. Due to the high cost of developing new drugs, rare diseases have historically been less attractive to pharmaceutical companies. Drug repurposing systematically leverages the detailed information available on approved drugs and reduces the time and money needed to deliver safe “new” treatments, but with greater success rates and a potentially more immediate impact on health care.
Read moreRepurposing Available Drugs to Treat Fragile X Syndrome – FRAXA Initiatives
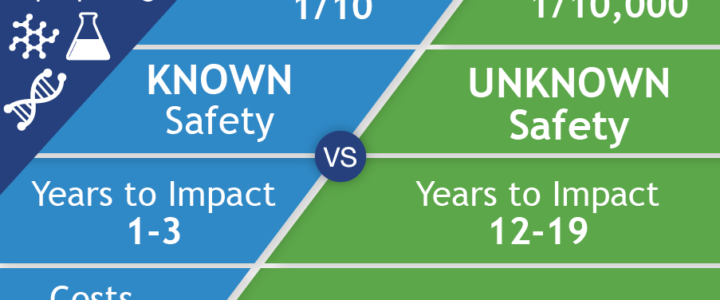
FRAXA Research Foundation was founded in 1994 to fund biomedical research aimed at finding a cure for Fragile X syndrome and, ultimately, autism. We prioritize translational research with the potential to lead to improved treatments for Fragile X in the near term. Our early efforts involved supporting a great deal of basic neuroscience to understand the cause of Fragile X. By 1996, these efforts had already begun to yield results useful for drug repurposing. To date, FRAXA has funded well over $25 million in research, with over $3 million of that for repurposing existing drugs for Fragile X.
Read more
