Celebrate the 5th World Fragile X Day on July 22! Join a global movement lighting up landmarks to raise awareness and support families affected by Fragile X.
Read moreAuthor: Holly Roos
19th Annual Fragile X Poker Run Raises $33,000 for Fragile X Research!

Friends, family, and supporters came together again at the Alleghany Inn in Sparta, NC, for the 19th Annual Fragile X Poker Run, continuing a powerful tradition started by organizers Jimmy and Dana Charlton.
Read moreWorld Fragile X Day 2024: A Global Celebration of Awareness and Research Progress

Recap of World Fragile X Day 2024, a global event by FRAXA that highlighted research advances and raised awareness for Fragile X syndrome. Explore beautiful photos of landmark illuminations around the world.
Read moreHole-in-One for Fragile X Research: Highlights from the 37th Annual Charity Golf Tournament

Celebrate the success of the Hall family’s golf tournament raising $35,000 for Fragile X research, contributing over $327,000 to date for FRAXA Research Foundation.
Read moreReactivating the Fragile X Gene in Young Mice Reverses Symptoms
A new FRAXA-funded research project offers hope that Fragile X syndrome could be treated by reactivating the gene which is shut down in people with the syndrome. Researchers at the University of California, Riverside report that they were able to reduce FXS symptoms by inserting the FMR1 gene into the brains of very young mice.
Read moreNew Fragile X Clinical Trial Announced by Healx

Healx’s AI-driven approach makes finding the right combination therapies more efficient, cost-effective, and rapidly ready for testing at FRAXA-DVI. It was this process that has brought Healx to its recent announcement sharing that it has received Investigational New Drug (IND) approval from the US Food and Drug Administration (FDA) for the Phase 2a clinical study of HLX-0201 (sulindac, an FDA-approved drug).
Read moreCorrecting the Brain’s Emotional Memory Center
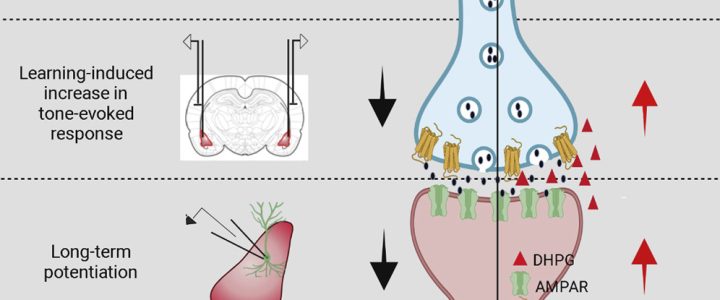
Cell Reports has published the results of the study “Correction of Amygdalar Dysfunction in Rat Model of Fragile X Syndrome” by FRAXA Investigator Dr. Sumatra Chattarji and his team at the National Centre for Biological Sciences in Bangalore, India. The researchers investigated the synaptic basis of deficient conditioned fear and its reversal in Fragile X syndrome rats.
Read moreBrain Organoids, Moving Fragile X Research Forward

There are many ways research produces discoveries, and all of them include a process of steps that build on each other. When an exciting new avenue appeared with potential for Fragile X syndrome, FRAXA stepped up to fund it. We now see the results of this grant and are excited to share them with you. The importance of different types of models have been shared and discussed over many years. We are now adding a “brain organoid” model to this group, and the potential behind it is really exciting.
Read morePromising Results of Preclinical Study of ANAVEX®2-73

We are excited to share that Anavex Life Sciences announced today that preclinical data of the ANAVEX®2-73 (blarcamesine) study in Fragile X syndrome were published in the peer-reviewed journal, Scientific Reports.
Read moreSynaptogenix Announced Intention to Launch a Fragile X Clinical Trial with Bryostatin

One of the most exciting kinds of work that FRAXA does is following the journey of an experimental new treatment until it is ready for trials in people with Fragile X. From an initial idea, through the development process, to clinical trials, FRAXA helps out all along the way. From an initial idea, through the development process, to clinical trials, FRAXA helps out all along the way. The recent announcement by Synaptogenix is a great example of how FRAXA funding and use of FRAXA-DVI can accelerate research on Fragile X.
Read more
