FRAXA Drug Validation Initiative (FRAXA-DVI)
The FRAXA Drug Validation Initiative (FRAXA-DVI) provides speedy, cost-effective, objective preclinical testing to validate investigational and repurposed compounds for Fragile X.
Help Rowan Thrive: Support Fragile X Research
Join the Gale and Kellogg families in supporting Fragile X research through FRAXA. Help Rowan and others thrive by funding life-changing treatments and advancing curative therapies.
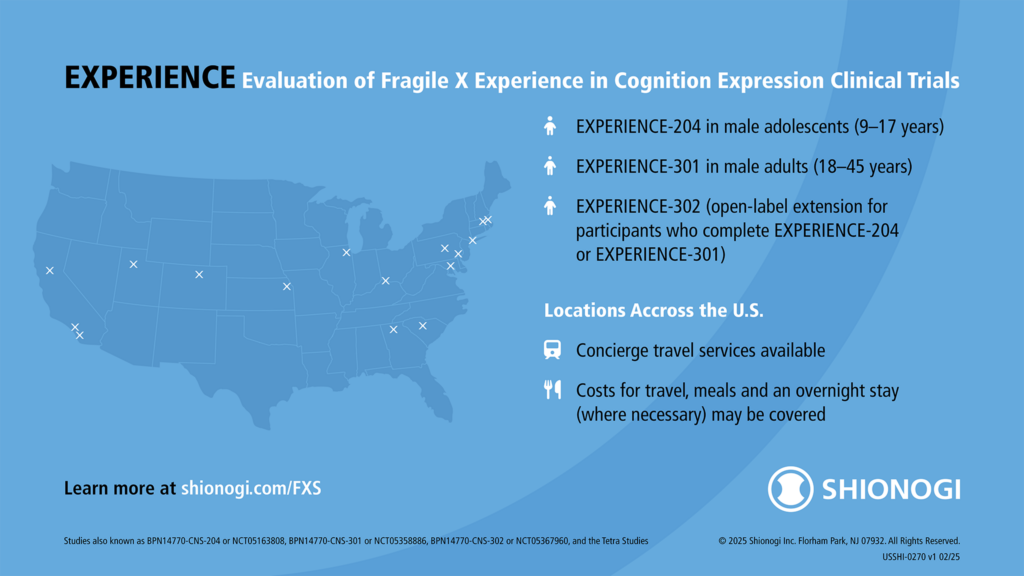
Shionogi’s EXPERIENCE Phase 3 Clinical Trial of Zatolmilast in Fragile X Syndrome
Learn more about Shionogi’s EXPERIENCE clinical trials for adults and adolescents with Fragile X syndrome, FRAXA’s role, and the open-label extension of these trials.
FRAXA-Funded Research Explores ISRIB as a Potential Treatment for Fragile X
ISRIB for Fragile X syndrome is being studied as a potential treatment to restore brain function and social behavior. Researchers investigate its effects.
Support the Stevenson Family Campaign
The march of time renews the commitment we made to a special needs community 25 years ago. We vowed to dream big and never give up until there were effective treatments available and eventually a cure for Fragile X syndrome, the most commonly inherited cause of intellectual disabilities and autism.
Screening Combinatorial Pharmacological Therapies for Fragile X Syndrome
This Stanford University team assessed combinatorial drug treatments to correct a broad spectrum of deficits observed in Fragile X syndrome. Results published.
Marvel Biosciences Partners with FRAXA to Test MB204 for Fragile X Syndrome
Marvel Biosciences and FRAXA Research Foundation are collaborating to test MB204, a promising new treatment for Fragile X which targets adenosine receptors.
BK Channel Openers: A New Drug for Fragile X Is Ready for Clinical Trials
A promising new BK channel opener, SPG601 from Spinogenix, is entering clinical trials for Fragile X syndrome. Learn about its potential to restore synaptic function and address core symptoms.
Fragile X Clinical Trial of New PDE4D Inhibitor from Tetra
A $200K FRAXA grant enabled a successful Phase 2 trial of a PDE4D inhibitor for adult men with Fragile X, showing strong cognitive gains without side effects or tolerance.
Contribution of Microglia to the Therapeutic Effects of Metformin and Adiponectin in Fragile X Syndrome
Why are some with Fragile X always hungry or overweight, yet rarely diabetic? This team is studying metabolism and testing treatments like metformin and diet.
Brain Organoids, Moving Fragile X Research Forward
FRAXA-funded scientists at Emory created human brain organoids that reveal Fragile X changes more clearly than mouse models, opening new paths to targeted treatments.
Synaptogenix Announced Intention to Launch a Fragile X Clinical Trial with Bryostatin
Bryostatin research has advanced from mouse models to human trials. Synaptogenix and Nemours make plans to test this potential treatment in Fragile X clinical trials.
Characterization of Microglia Transcriptional Profile in Fmr1 Knockout Mice Model
Microglia are excessively activated in Fragile X models. The team will investigate the mechanisms and attempt to correct this using drugs.
Bryostatin-1 in Long-term Use Seen to Arrest Fragile X Symptoms in Mouse Model
Long-term, but not short-term, treatment with bryostatin-1 — Neurotrope’s lead investigational therapy — arrested such behavioral and cognitive symptoms as hyperactivity, difficulties with daily life activities, and learning and memory deficits in a mouse model of Fragile X syndrome.
Positive Results Reported in Phase II Fragile X Clinical Trial of PDE4D Inhibitor Zatolmilast from Tetra Therapeutics
Today, Tetra Therapeutics announces the first unequivocally positive phase 2 clinical trial in Fragile X syndrome, press release below. The results do not depend on carving out a subset of patients or post hoc analysis.
Scientists Find a New Way to Reverse Symptoms of Fragile X
FRAXA Investigator and MIT Professor Mark Bear and his colleagues have identified a valuable new target for Fragile X therapeutics: GSK3 alpha. Several FRAXA research teams previously identified GSK3 beta as a treatment target for Fragile X. The catch is that, so far, GSK3 beta inhibitors have proven too toxic for regular use. Dr. Bear’s new discovery opens up the possibility of developing more selective compounds with less toxicity and fewer side effects. Interestingly, lithium inhibits both GSK3 versions – alpha and beta.
Companies Move to Advance Potential Cognitive Treatment for Fragile X
Tetra Therapeutics and Shionogi announced plans to expand their partnership supporting BPN14770, a treatment candidate for disorders marked by cognitive and memory deficits, including Fragile X syndrome and Alzheimer’s disease. The agreement builds on an earlier collaboration between the two companies, and aims to further accelerate BPN14770’s development and potential marketing. It is currently in clinical testing in both Fragile X and Alzheimer’s patients.
Ketogenic Diet Eases Symptoms in Fragile X Male Mice
Westmark Lab is studying sleep patterns in Fragile X mice, working with UW-Madison to develop tools that speed EEG data analysis
FRAXA Biotech Games, It Can Only Happen in an Open Community
The FRAXA Biotech Games lit up Cambridge Crossing with biotech teams uniting to raise funds and awareness for Fragile X research.
Tetra Announces $40M to Advance BPN14770 for FXS and Alzheimer’s Disease
Tetra Discovery Partners has signed a multi-part deal that could bring it up to $160 million, plus royalties, from Shionogi & Co, Ltd, a Japanese major research-driven pharmaceutical company. Tetra currently is conducting an investigational Phase 2 study of BPN14770 in adults with Fragile X Syndrome, an indication for which BPN14770 has received Orphan Drug Designation from the US Food and Drug Administration. This clinical trial was made possible by early work with the FRAXA-DVI and over $200,000 from FRAXA.
Metformin and Aberrant Insulin Signaling in a Fragile X Mouse Model
FRAXA-funded research is revealing how insulin signaling is altered in Fragile X and whether lowering it, including with metformin, could ease symptoms.
Mega Green Tea Extract to Treat Fragile X?
Green tea is thought to have many benefits, particularly in cognitive function. In 2012-14, FRAXA Research Foundation funded a clinical trial to assess the effects of EGCG (green tea extract) on cognitive function in adults with FXS. Drs. Rafael de la Torre and Mara Dierssen Sotos, principal researchers in Barcelona, Spain, reported memory, attention, and mental flexibility improvements.
Tetra Discovery Partners Initiates Phase 2 Trial of BPN14770 in Fragile X Syndrome
This 2-Period Crossover Study of BPN14770 is accepting adults males with Fragile X syndrome at Rush University Medical Center in Chicago. Principal Investigator of the study is Elizabeth Berry-Kravis, MD, PhD.
A selective inhibitor of the phosphodiesterase type-4D (PDE4D), BPN14770 has shown the ability to improve the quality of connections between neurons and to improve multiple behavioral outcomes in the Fragile X mouse model.