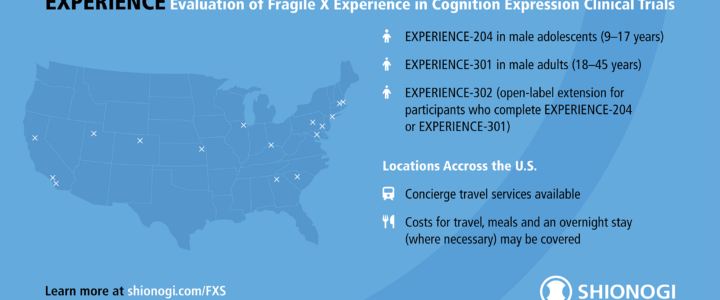
Shionogi’s EXPERIENCE clinical trials for Fragile X syndrome are nearing completion. Enrollment for the adult trial (EXPERIENCE-301) is now closed, while the adolescent trial (EXPERIENCE-204) is in its final phase. Learn more about the study, FRAXA’s role, and the open-label extension.
Read moreTrials and Studies
Harmony Biosciences Phase 3 Clinical Trial (RECONNECT) with At-Home Option

Harmony Biosciences is now recruiting for the RECONNECT Phase 3 clinical trial of ZYN002, a potential treatment for Fragile X syndrome. The trial offers an at-home participation option, making it accessible for patients across the US, Australia, and the UK. Join us in this groundbreaking study and contribute to the future of Fragile X syndrome treatment.
Read moreFRAXA Research Foundation Joins COMBINEDBrain Consortium for Fragile X Biomarker Research
Help accelerate research on Fragile X syndrome biomarkers by contributing samples to the COMBINEDBrain Consortium’s project. Contact Katie Clapp at FRAXA Research Foundation to learn how you can participate.
Read morePharmacologically Activating mGluR7 as a Novel Therapy for Fragile X Syndrome
Join Dr. Tsai and Dr. Kumar on a journey into novel treatment avenues for Fragile X syndrome. Learn how activating mGluR7 could be a game-changer, opening up uncharted therapeutic territory.
Read moreRecruiting: Unveiling Probiotic Potential in Fragile X Syndrome Clinical Trial

First of its kind in Serbia, this clinical trial explores probiotic intervention as a potential treatment avenue for Fragile X syndrome.
Read moreFragile X Clinical Trial of New PDE4D Inhibitor from Tetra

With a $200,043 grant from FRAXA Research Foundation, Dr. Elizabeth Berry-Kravis completed a successful Phase 2 clinical trial of a PDE4 inhibitor for adult men with Fragile X syndrome. This trial treated 30 males, 18-45 years of age with a new PDE4D allosteric inhibitor from Tetra Discovery Partners using a crossover design, so that everyone got active drug for part of the time and placebo for part of the time.
Read moreRecruiting: BRIDGE Study (BRain Indicators of Developmental Growth)
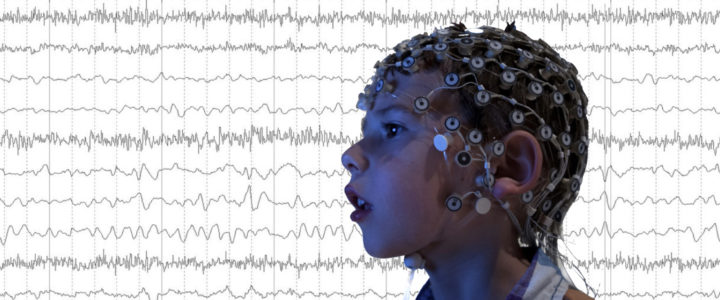
This study from the Wilkinson Lab at Boston Children’s Hospital is investigating how differences in brain activity affect learning, language and behavior in children with Fragile X syndrome, Down syndrome, and Autism Spectrum Disorder. One of the goals is to find brain markers that predict cognitive, language, and behavioral difficulties in these groups. Another goal is to better understand the differences in brain activity between young children with and without Fragile X and Down Syndrome, and whether these differences are similar in children with Autism Spectrum Disorder.
Read moreRecruiting: Clinical Study of Non-Invasive EEG for Children Ages 2-7

Dr. Carol Wilkinson, MD PhD, and Dr. Charles Nelson, PhD, at Boston Children’s Hospital are recruiting children ages 2-7 years with Fragile X syndrome to participate in a study of brain differences using non-invasive EEG.
Read moreLovamix: Clinical Trial of Combined Treatment of Minocycline and Lovastatin in Fragile X Syndrome

With a $66,714 grant from the FRAXA Research Foundation awarded over 2015-2017, Dr. Francois Corbin at the Universite of Sherbrooke will test the safety and synergistic effects of lovastatin and minocycline in patients with Fragile X syndrome.
Read moreFX-Learn Clinical Trial for Children with Fragile X

Thirteen centers across the US enrolled children with Fragile X in a large-scale clinical trial of Novartis AFQ056. Dr. Elizabeth Berry-Kravis and colleagues aim to show that this targeted treatment — an mGluR5 blocker for Fragile X which failed in previous adult human trials — can be better evaluated by studying effects on learning in young children.
Read morePurposeful and FRAXA Partnership Leads to Clinical Trial

Can a combination of drugs make a meaningful difference for people with Fragile X? A new clinical trial is going to find out. 15-20 adult men with Fragile X will be included in this trial to test the effects of an available drug and a nutritional supplement taken together.
Read morePositive Results Reported in Phase II Fragile X Clinical Trial of PDE4D Inhibitor Zatolmilast from Tetra Therapeutics

Today, Tetra Therapeutics announces the first unequivocally positive phase 2 clinical trial in Fragile X syndrome, press release below. The results do not depend on carving out a subset of patients or post hoc analysis.
Read morefNIRS to Measure Treatment Response in Young Children with Fragile X

FRAXA Research Foundation has awarded a $90,000 research grant to Dr. Craig Erickson and Dr. Elizabeth Smith at Cincinnati Children’s Hospital to test functional near-infrared spectroscopy (fNIRS), in children who have Fragile X syndrome. fNIRS is safe, non-invasive, and easily-tolerated. It uses light sources and sensors on the scalp to build a heat map of the brain in action.
Read moreClinical Trial of Metformin for Fragile X Syndrome

Metformin is commonly prescribed to control high blood sugar in type 2 diabetes. With a $50,000 grant from FRAXA Research Foundation, Dr. Artuela Çaku and Dr. Francois LePage are conducting an open-label clinical trial of metformin for children and adults with Fragile X syndrome, at the University of Sherbrooke in Canada.
Read moreAripiprazole as a Treatment for Fragile X Syndrome

Many medications are used to help people with Fragile X cope. But few clinical trials have been done on these drugs. Years ago FRAXA funded Dr. Craig Erickson to run a trial of aripiprazole (aka Abilify). FRAXA guest writer Hannah Miles recently caught up with Dr. Erickson to learn the results of the trial.
Read moreFragile X Clinical Trial of AZD7325 in Adults

With a $51,000 grant from FRAXA Research Foundation, Dr. Craig Erickson conducting a double-blind, placebo-controlled clinical trial of AZD7325 in adults ages 18-50 with Fragile X syndrome at Cincinnati Children’s Hospital. The compound being studied is an investigational new drug from AstraZeneca that targets GABA (A) receptors.
Read moreClinical Trial of Ganaxolone in Patients with Fragile X Syndrome

With a $90,000 grant from FRAXA Research Foundation funded during 2014-2015, Dr. Frank Kooy and colleagues at the University of Antwerp are conducting a double blind crossover trial of ganaxolone in patients with Fragile X syndrome. Results of this study were mixed (see Marinus: Results from Phase 2 Exploratory Clinical Study Support Continued Development of Ganaxolone in Fragile X Syndrome.)
Read moreNew Fragile X Clinical Trial for Children Launching in June 2017

Rush University Medical Center Professor Elizabeth M. Berry-Kravis, MD, PhD, has launched and is recruiting participants for a large-scale clinical trial to study effects of AFQ056, an mGluR5 blocker, on learning in young children.
Read moreDouble Down: Fragile X Clinical Trial Combines Two Available Drugs
If all the science world’s a stage, Fragile X researchers are more than merely players. They are center stage. So believes Francois Corbin, MD, PhD, professor, Université de Sherbrooke, Canada, who directs the university’s Fragile X Clinic. Corbin, who has received more than $100,000 in FRAXA support since 2012, is leading a pilot randomized Phase II trial, exploring the tolerability and the synergistic effect of a combined therapy.
Read moreNeuren’s Tofinetide Successful in Phase 2 Clinical Trial in Fragile X
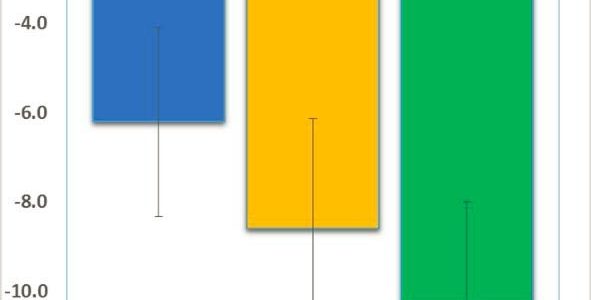
We are pleased to share great news adapted from Neuren’s press release: Neuren’s phase 2 trial has successfully established proof of concept and provides a strong rationale for Neuren to move forward with developing trofinetide for Fragile X syndrome. In this initial small trial with a relatively short treatment period, trofinetide was very well tolerated, with the high dose (70 mg/kg twice daily) demonstrating a consistent pattern of clinical improvement, observed in both clinician and caregiver assessments.
Read moreNeuren’s NNZ-2566 Shows Clinical Benefit in Rett Syndrome Trial
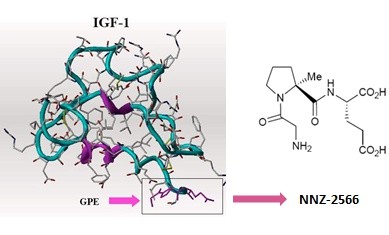
This isn’t a Fragile X trial, but the Neuren compound, NNZ-2566, that is in trials now for Fragile X has shown significant positive effects in a Phase 2 trial for Rett syndrome. The results of the trial are interesting, in that improvement was seen a Rett syndrome-specific rating scale compared to placebo, and there was also improvement noted on the CGI-I (Clinical Global Impression of Improvement) and Caregiver Top 3 Concerns. However, there was no effect seen on ABC scores (Aberrant Behavior Checklist) compared to placebo. Many in the Fragile X field have noted the inadequacies of the ABC; indeed, it was never designed or intended to be an outcome measure for clinical trials.
Read morePhase 1 Clinical Trial of Mega Green Tea Extract in Fragile X Syndrome

With a $124,000 grant from the FRAXA Research Foundation from 2012-2014, Dr. Mara Dierssen and Dr. Rafael de la Torre conducted preclinical studies in Fragile X knockout mice and a clinical trial in Fragile X patients using Mega Green Tea Extract, which contains 45% by weight epigallocatechin gallate (EGCG).
Read moreRoche reports clinical trial negative results

Roche has shared the sad news that their clinical trials in Fragile X have been unsuccessful. They will host a Webcast on Thursday, September 18, from 12:30pm – 1:30pm (EDT) to explain the results. For details and dial-in information please see this letter from Luca Santarelli, the
Read moreWhy Did Fragile X Clinical Trials of mGluR Antagonists Fail?

by Michael Tranfaglia, MD. In my opinion, the Fragile X clinical trials of AFQ056 sponsored by Novartis failed because of a dose range that was inadequate for Fragile X, and because of the unexpected development of tolerance.
Read moreFragile X Clinical Trial: Novartis Trial Results Are In, and They’re Not Pretty
This year’s Gordon Conference just finished, and Novartis presented their results for the first time (though advisors and advocates had been given a private peak months ago.) To say that the trial results for AFQ056 were disappointing would be the understatement of the century!
Read more