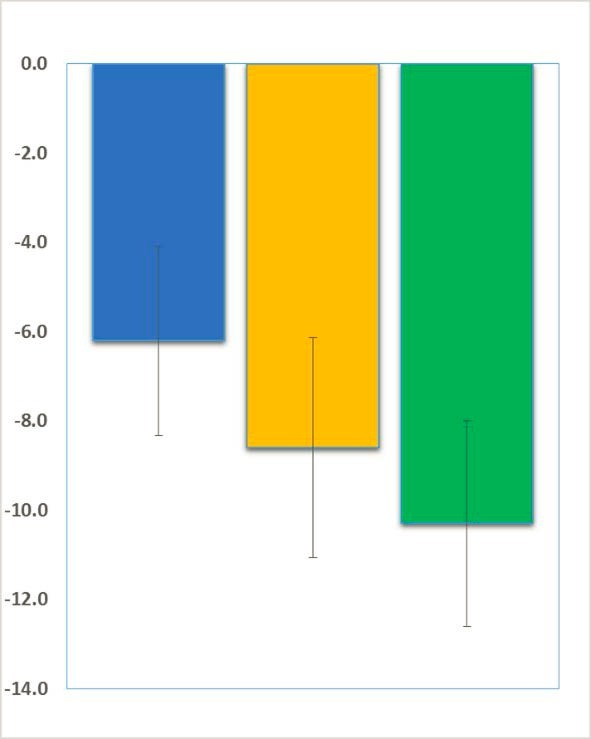
Positive Results in Fragile X Syndrome and Rett Syndrome
Beneficial effects of trofinetide have now been observed in both Fragile X syndrome and Rett syndrome. Neuren announced encouraging results of their phase 2 trofinetide trial in Rett Syndrome last year.
Trofinetide Tested at FRAXA’s Drug Validation Initiative
FRAXA’s Drug Validation Initiative, run by Dr. Patricia Cogram in Santiago, Chile, enabled Neuren to quickly and efficiently test their molecule in Fragile X knockout mice. In fact, preliminary results in the Fragile X mice were even more promising then they were in Rett Syndrome, which was the original target for this drug, and those results launched Neuren’s Fragile X program.
See NNZ-2566, a novel analog of (1-3) IGF-1, as a potential therapeutic agent for Fragile X syndrome and www.fraxa.org/funded-research/cogram/
“Neuren is immensely grateful for the contribution that the Fragile X community has made to support our research endeavor. This type of clinical trial research is only possible because of the people with Fragile X and their families who devote their time and energy to participate in the study and the dedicated and knowledgeable clinicians who implement it. Moreover, FRAXA has been instrumental to this clinical trial both in providing practical support with outreach and enrollment, but also in providing insight on ways to better ensure outcomes are meaningful for families.”
Nancy E. Jones, PhD, Neuren Pharmaceuticals
Next Steps
Based on these results and feedback from clinical experts in Fragile X syndrome, Neuren is strongly encouraged to advance to the next step in clinical development. This will likely involve a study in younger children with Fragile X syndrome and may examine a longer treatment duration with higher doses. This next study will also refine the outcome measures that may be used in a Phase 3 study (clinical trial phases explained here).

