Explore how the FRAXA Drug Validation Initiative is revolutionizing Fragile X syndrome treatment, leading the charge towards innovative therapies and hope for affected families.
Read moreminocycline
Lovamix: Clinical Trial of Combined Treatment of Minocycline and Lovastatin in Fragile X Syndrome

With a $66,714 grant from the FRAXA Research Foundation awarded over 2015-2017, Dr. Francois Corbin at the Universite of Sherbrooke will test the safety and synergistic effects of lovastatin and minocycline in patients with Fragile X syndrome.
Read moreDrug Repurposing for Rare Disease and the Future of Health – The Genetics Podcast

In this double-bill episode of The Genetics Podcast, Dr. Patrick Short talks to two key rare disease researchers in the field: Dr. Bruce Bloom, CCO of Healx, and Dr. Mike Tranfaglia, CSO of FRAXA. Both draw on their wide-ranging personal and professional experiences to discuss the successes and opportunities of drug repurposing, the power of using machine learning, and the work they’ve been doing to aid in finding effective treatments for Fragile X.
Read moreConsidering Available Drugs for Fragile X: My Favorite Combination (So Far)

Which of the available drugs are best for fragile X? We tend to think of drugs according to their primary activity in the body, but very few drugs are totally selective and specific. There are differences between drugs in any given class, and these differences may be critical. Most drugs have “off-target” effects which are usually considered side effects, and it is these side effects which can have key advantages, in some cases.
Read moreResults Reported: Using EEG Responses to Sound for Fragile X Drug Discovery

Jonathan Lovelace, a FRAXA funded Postdoc at UC Riverside, has made some exciting EEG findings over the past few years studying auditory hypersensitivity in mice and therapeutic drug treatments. A big obstacle in FXS research has been establishing reliable, unbiased, and translation relevant biomarkers that can be used to determine the effectiveness of therapies. One of the most important discoveries they have made is the striking similarity in EEG biomarkers between mice and humans.
Read moreHealx Raises $56M to use AI to Find Treatments for Fragile X & Other Rare Diseases

Healx has secured $56M in new financing to build a clinical-stage portfolio for rare diseases, including treatments for Fragile X syndrome, and to launch a global Rare Treatment Accelerator program. Where the traditional drug discovery model takes more than a decade and can run into the billions of dollars, Healx’s AI-driven approach makes the process faster, more efficient and cost-effective.
Read moreDrug Duo Delivers Brain, Behavioral Benefits for Fragile X Syndrome

Administering a cholesterol drug alongside an antibiotic eases atypical behavior and restores the signaling balance in the brains of people with fragile X syndrome.
Read moreUnderstanding and Reversing Hypersensitivity to Sounds in Fragile X Syndrome

With a $90,000 grant from FRAXA Research Foundation over 2018-2019, Drs. Devin Binder, Iryna Ethell, and Patricia Pirbhoy at the University of California at Riverside aim to understand – and reverse – hypersensitivity to sound in Fragile X syndrome.
Read moreIn Their Own Words: Reports From the International Fragile X Workshop

The 18th International Fragile X and Related Neurodevelopmental Disorders Workshop in Quebec, Canada, was a great success, featuring Fragile X much more heavily than any previous meeting in this series! We asked our speakers to summarize their work in their own words, with brief updates from researchers investigating Fragile X.
Read moreCombinatorial Drug Treatment in a Model of Fragile X Syndrome using Novel Biomarkers

With a $90,000 grant from FRAXA Research Foundation awarded over 2016-2017, University of California researchers Khaleel Razak, PhD, and Jonathan W. Lovelace, PhD, are exploring drug combinations to limit hypersensitivity to sounds in Fragile X mice.
Read moreFRAXA Wins Award for Drug Repurposing

Cures Within Reach, the leading global nonprofit focused on repurposing research as a fast track to saving patient lives, has awarded FRAXA Research Foundation the 2017 Golan Christie Taglia Patient Impact Philanthropy Award for efforts to find treatments for the rare disease Fragile X syndrome.
Read moreRepurposing Available Drugs to Treat Fragile X Syndrome – FRAXA Initiatives
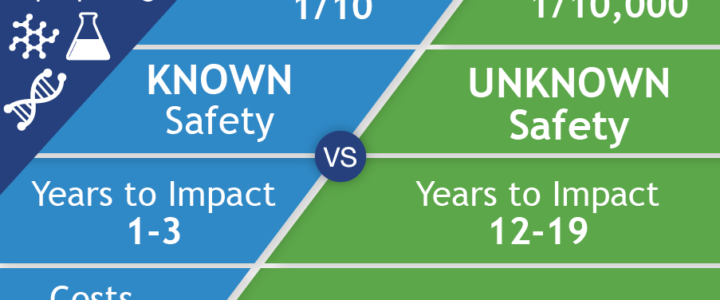
FRAXA Research Foundation was founded in 1994 to fund biomedical research aimed at finding a cure for Fragile X syndrome and, ultimately, autism. We prioritize translational research with the potential to lead to improved treatments for Fragile X in the near term. Our early efforts involved supporting a great deal of basic neuroscience to understand the cause of Fragile X. By 1996, these efforts had already begun to yield results useful for drug repurposing. To date, FRAXA has funded well over $25 million in research, with over $3 million of that for repurposing existing drugs for Fragile X.
Read moreDouble Down: Fragile X Clinical Trial Combines Two Available Drugs
If all the science world’s a stage, Fragile X researchers are more than merely players. They are center stage. So believes Francois Corbin, MD, PhD, professor, Université de Sherbrooke, Canada, who directs the university’s Fragile X Clinic. Corbin, who has received more than $100,000 in FRAXA support since 2012, is leading a pilot randomized Phase II trial, exploring the tolerability and the synergistic effect of a combined therapy.
Read moreWhy Did Fragile X Clinical Trials of mGluR Antagonists Fail?

by Michael Tranfaglia, MD. In my opinion, the Fragile X clinical trials of AFQ056 sponsored by Novartis failed because of a dose range that was inadequate for Fragile X, and because of the unexpected development of tolerance.
Read moreFragile X Syndrome Treatment Target: MMP-9

Dr. Ethell was awarded FRAXA Research Foundation funding from 2008-2011 and 2012-present. This latest work shows that human Fragile X tissues have elevated levels of the extracellular enzyme MMP-9, as well as an increase in the active fraction of that protein (like most enzymes, MMP-9 can exist in an inactive form which can be switched on rapidly; this kind of regulation is important in most biological pathways.)
Read moreFragile X Clinical Trial: Novartis Trial Results Are In, and They’re Not Pretty
This year’s Gordon Conference just finished, and Novartis presented their results for the first time (though advisors and advocates had been given a private peak months ago.) To say that the trial results for AFQ056 were disappointing would be the understatement of the century!
Read moreSocial Behavior as an Outcome Measure for Fragile X Clinical Trials

One of the features of the Fragile X mouse model which is relevant to the human Fragile X syndrome (and autism) is social behavior. Several tests show consistent social behavioral abnormalities in the Fragile X mouse model. With a $140,000 grant from FRAXA Research Foundation in 2012-2013, Dr. Willemsen at Erasmus University used social behavior tests to measure the effectiveness of several drug strategies.
Read moreMatrix Metalloproteinase Therapeutic Treatments for Fragile X Syndrome

With a $157,000 grant from the FRAXA Research Foundation in 2012-2013, Dr. Kendal Broadie and Dr. Cheryl Gatto worked to define the distinct but also overlapping roles for MMP-1 and MMP-2 in synaptic structural and functional development. In drug studies with Fragile X fruit flies, they will be testing a range of MMPIs in drug treatments to compare effectiveness during development and at maturity, in order to define the contributions of FXS developmental impairments and adult recovery/plasticity.
Read moreLovastatin Discovery in Fragile X Mice Leads FRAXA to Fund Clinical Trials

Dr. Emily Osterweil was awarded the FRAXA Pioneer Award at the opening dinner of the 2011 FRAXA Investigators Meeting in Southbridge, MA for her work demonstrating that Lovastatin could treat Fragile X. Dr. Osterweil conducted her experiments in the MIT laboratory of Dr. Mark Bear and has since established her own laboratory at the University of Edinburgh. The team discovered that lovastatin, a drug widely prescribed for high cholesterol, can correct excess hippocampal protein synthesis in the mouse model of FXS and can prevent epileptogenesis. The work is published in the prestigious neuroscience journal Neuron: Lovastatin Corrects Excess Protein Synthesis and Prevents Epileptogenesis in a Mouse Model of Fragile X Syndrome.
Read moreEffects of minocycline on vocal production and auditory processing in a mouse model of Fragile X

With $135,000 in grants from FRAXA Research Foundation over several years, Dr. Khaleel Razak and Dr. Iryna Ethell explored robust biomarkers relevant to the FXS and the efficacy of minocycline treatment.
Read moreClinical Trials FAQ ← Frequently Asked Questions
Question: How Do Families Decide Which Trial is Best for Them? Answer: Each of the trials has different requirements for joining, so many – if not most – people will only be eligible for one trial after screening. The best way to approach this is to call the clinic contact closest to your area and discuss this with him/her. Age, weight, current medications, behavior, and IQ are all factors.
Read moreWhat Works, and What Doesn’t
At the start, it’s always hard to know what methods will work best for something as complex as the development of disease-modifying treatments for Fragile X. But, we’ve always tried to let the science lead us down the right path. At this point, the results are unequivocal, and they have shaped how we are looking for the Next Great Thing in Fragile X treatments.
Read morePilot Clinical Trial of Lithium in Fragile X Shows Promising Results

With a $65,000 grant from FRAXA Research Foundation in 2005, Dr. Berry-Kravis at the Rush University Medical Center conducted a pilot clinical trial of lithium in 15 patients with Fragile X syndrome. Results published.
Read moreEncouraging Results from First Trial of Minocycline in Fragile X

With a $40,000 grant from FRAXA, Dr. Carlo Paribello and his team at the Surrey Place Centre Fragile X clinic in Toronto, Ontario, ran an open label trial to see if minocycline can improve learning and reduce anxiety and behavioral problems in people with Fragile X. Twenty participants between the ages of 13 and 35 years took minocycline for two months.
Read moreRole of Matrix Metalloproteinases (MMP-9) in Fragile X

With a $220,000 grant from FRAXA Research Foundation over 3 years, Dr. Iryna Ethell from the University of California at Riverside studied the regulation of dendritic structure by matrix metalloproteinases and other extracellular signaling pathways. This work identified a major treatment strategy for Fragile X with the available MMP-9 inhibitor, minocycline.
Read more
