Fragile X Research Tackles High Anxiety – Peter Vanderklish
Yes, we all know the signs of Fragile X anxiety: Ears begin turning red followed by incessant pacing, heavy breathing, stiffening body, flapping, jumping, avoidance or yelling. Sometimes, it’s the more severe screaming, pinching, scratching, biting and general tearing things up or, worse, the nuclear meltdown.
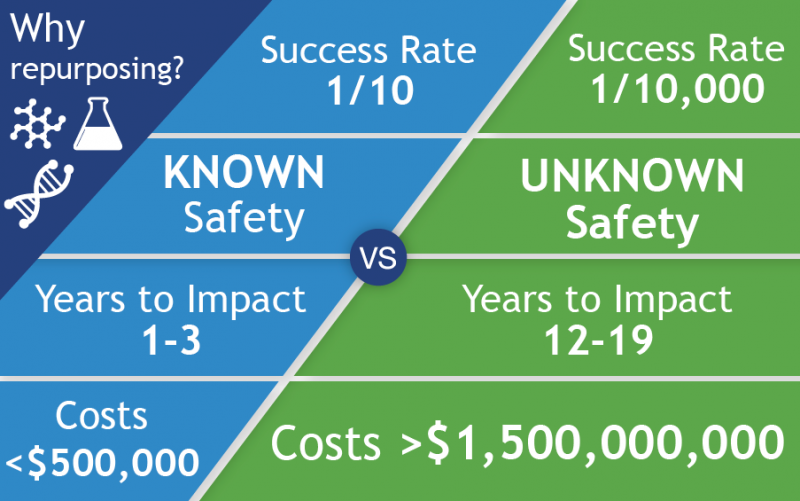
University of Cambridge Startup Healx is Rapidly Identifying Existing Drugs to Help Fragile X Patients
FRAXA awarded $44,000 to Healx in 2017 for drug repurposing to find new treatments for Fragile X syndrome. The results of this study include eight top "hits" which show promise for Fragile X. FRAXA is further investigating these hits.
Trial and No Error: Better Outcomes for Clinical Trials in Fragile X Syndrome
Johns Hopkins researcher Christina Timmerman, PhD, searches for a less subjective method to determine if a drug is working in patients with Fragile X syndrome. Many parents of children with Fragile X syndrome were crushed when promising drug trials were unexpectedly stopped a few years ago because subjective behavior-based outcome measures did not justify continuing the trials. The strong feelings linger today. If all goes well with Christina Timmerman’s research, future drug trials may be able to continue with additional metrics for assessment, until there are advanced treatments or even a cure for Fragile X syndrome.
NIH Investigator Carolyn Beebe Smith, PhD, Looks to Improve Sleep in Fragile X Syndrome
Our sons with Fragile X Syndrome typically go to bed early and rise early. Sometimes they jump on us while we are sleeping at 3 a.m., excited to start their day. For heaven’s sake, why? The answer may come from Carolyn Beebe Smith, PhD, senior investigator, Section on Neuroadaptation and Protein Metabolism, National Institute of Mental Health, National Institutes of Health, Bethesda, Maryland. She is studying why children, in particularly boys, with FXS have problems sleeping.
New Fragile X Clinical Trial for Children Launching in June 2017
Rush University Medical Center Professor Elizabeth M. Berry-Kravis, MD, PhD, has launched and is recruiting participants for a large-scale clinical trial to study effects of AFQ056, an mGluR5 blocker, on learning in young children.
Mark Bear’s Goal: Disease-Modifying Treatments for Fragile X
Researcher Mark Bear, PhD, Picower Professor of Neuroscience, sees success developing disease-modifying treatments for Fragile X syndrome and other developmental brain disorders. Finally, hope. And it comes from his lab, The Picower Institute for Learning and Memory, Department of Brain and Cognitive Sciences, Massachusetts Institute of Technology.
Kimberly Huber, PhD, Explores Hyperexcitability in Fragile X Syndrome
What causes hyperexcitability? Dr. Kimberly Huber seeks to understand how FMRP regulates connections between brain cells and the function of brain circuits.
Identifying Biomarkers for Fragile X Syndrome – A Study in Argentina
Bio·mark·er, noun, a distinctive biological or biologically derived indicator of a process, event, or condition. Doesn’t help? Well, it’s perfectly clear to Argentinian researchers Patricia Cogram, PhD, and Paulina Carullo, MD, from the FLENI Institute in Buenos Aires, Argentina. They understand there is an urgent need for validated biomarkers after recent Fragile X syndrome clinical trials have failed on their primary endpoints.
Fulcrum Therapeutics Launched with $55 Million to Reactivate the Fragile X Gene
A new company has launched that will invest tens of millions in reactivating the Fragile X gene. With $55 million in investment funds, Fulcrum Therapeutics in Cambridge, MA, aim to develop small molecules to control gene expression. These potential new treatments would be based on controlling genetic on- and off-switches of disease genes. Fulcrum will start with two diseases: Fragile X syndrome and a rare form of muscular dystrophy. FRAXA is funding one of the founding scientists, Jeannie Lee, MD, PhD, of Harvard University, and has been working with others on the new Fulcrum team.
Cornell University Researcher Looks to Restore Fragile X Protein in Neurons
Which is the right FMRP for therapeutic development of Fragile X syndrome? When researchers develop effective drugs that reactivate FMRP — the protein that is normally silenced in Fragile X — what in the world will they do next? So ponders Cornell University researcher Samie R. Jaffrey, MD, PhD. Jaffrey, professor, Pharmacology, Weill Cornell Medical College, Cornell University, knows reactivating FMRP will lead to many important questions, such as: Which cell type needs FMRP? How much FMRP protein is needed to restore brain function? Where in the brain will FMRP protein be needed? Where in a neuron will the FMRP needs to be expressed?
Memory Lane: New Research to Improve Memory in Fragile X Mice
University of Texas at Austin Researchers Daniel Johnston, PhD, and Jennifer J. Siegel, PhD, explore ways to Iimprove memory in Fragile X mice.
Achieving Predictability: Developing Biomarkers for Fragile X Patients
New York University scientists make progress developing biomarker signatures and cataloging the types of Fragile X patients who will most likely benefit from new therapies. Take a closer look at your son or daughter with Fragile X syndrome. If you meet another child with Fragile X syndrome, chances are he/she may seem totally different to you, yet everyone is united under a FXS diagnosis. Discovering the biological reasons behind these differences is key to identifying which children will respond to what treatment. But how do you find the ‘prediction formula’? New York University scientists may soon know.
University of Michigan researcher Peter Todd, MD, PhD, Aims to Selectively Turn the Fragile X Gene Back on in Human Cells
Fish like salmon are born in fresh water streams and rivers. When the time comes for them to breed, they return to the stream of their birth to lay eggs in the same spot where they were born. To accomplish this, they must swim upstream against the current or flow of the stream. Taking a page out of the salmon DNA playbook, University of Michigan scientists Peter Todd, MD, PhD, and postdoctoral fellow Jill Haenfler, Ph.D., are exploring unchartered waters to find a cure for Fragile X Syndrome. The researchers are adapting CRISPR research to reactivate the FMR1 gene, which provides instructions for making a protein called FMRP — needed for normal brain development.
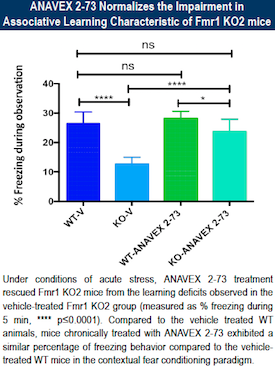
New compound from Anavex Improves Learning and Behavior in Fragile X Mice
The investigational drug Anavex 2-73 was able to improve intellectual, learning and hyperactivity measures in a mouse model of Fragile X syndrome. This is a sigma-1 receptor agonist being developed for autism spectrum disorders and Alzheimer’s disease.
Meltdown no more? Targeting Hypersensitivity in Fragile X
Meet Khaleel Razak, PhD, and Jonathan W. Lovelace, PhD, FRAXA-funded researchers at University of California, Riverside who are tackling Fragile X.
Researcher David Nelson, PhD, Explores New Cell Strategies for Fragile X Syndrome, FXTAS and FXPOI
It’s rare to find a researcher working on the Big Three — Fragile X Syndrome (FXS), Fragile X-associated Tremor/Ataxia Syndrome (FXTAS) and Fragile X-associated primary ovarian insufficiency (FXPOI). Then again, David Nelson, PhD, is the rare bird. Nelson is a professor of Molecular and Human Genetics, Baylor College of Medicine, and director of Baylor’s Graduate Program in Integrative Molecular and Biomedical Sciences. He has been involved in FXS research since the late 1980s where he helped identify the mutation and the FMR1 gene. These days, researchers in Nelson’s lab at Baylor are studying FXS, FXTAS and FXPOI using mouse models.
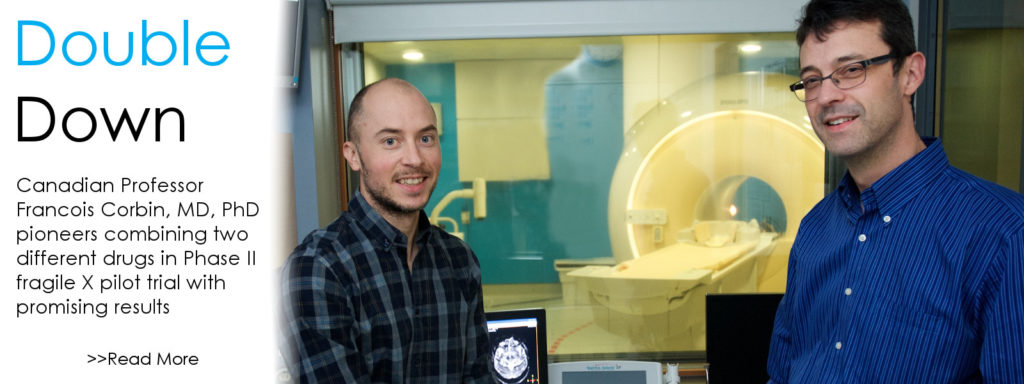
Double Down: Fragile X Clinical Trial Combines Two Available Drugs
If all the science world’s a stage, Fragile X researchers are more than merely players. They are center stage. So believes Francois Corbin, MD, PhD, professor, Université de Sherbrooke, Canada, who directs the university’s Fragile X Clinic. Corbin, who has received more than $100,000 in FRAXA support since 2012, is leading a pilot randomized Phase II trial, exploring the tolerability and the synergistic effect of a combined therapy.
The X Factor – Turning on X Chromosome Genes to Treat X-linked Disorders
Harvard researcher Jeannie T. Lee, MD, PhD, moves closer to turning on select genes on the X chromosome to treat people with X-linked disorders.
Fragile X Fruit Fly Research Bears Fruit
A new FRAXA-funded study shows how the hormone insulin – usually associated with diabetes — is involved in the daily activity patterns and learning deficits in the fruit fly model of Fragile X Syndrome (FXS). The study also reveal a metabolic pathway that can be targeted by new and already approved drugs to treat Fragile X patients, notably metformin.
Fragile X Cure One Step Closer with FRAXA Support of $1 Million in New Research
4 Countries – 10 Teams – $1 Million for finding new treatment targets, to pinpointing outcome measures for future clinical trials, to attempting to reactivate the gene which is silenced in Fragile X syndrome, these innovative scientists will bring us closer to a cure.
Can STEP Inhibitors Treat Fragile X Syndrome? Yale Professor Investigates
Yale Professor Paul Lombroso, MD, is testing STEP inhibitors to improve cognitive and social behaviors in those affected by Fragile X syndrome.
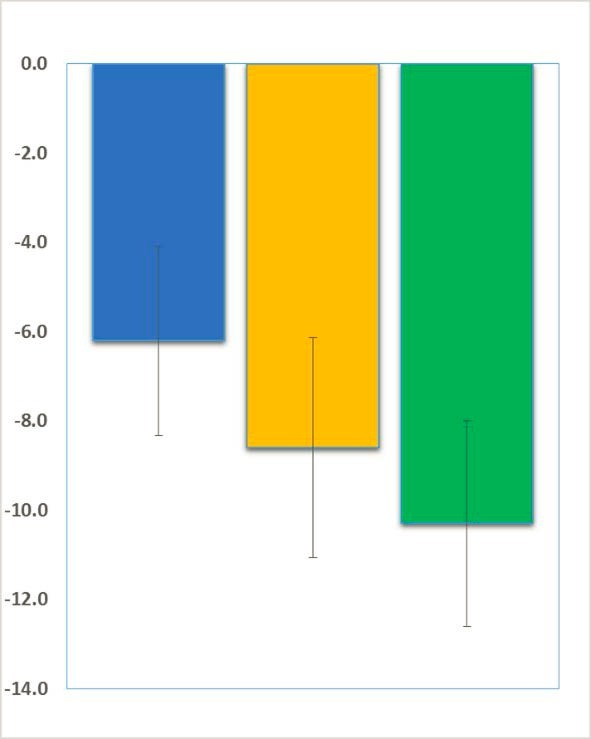
Neuren’s Tofinetide Successful in Phase 2 Clinical Trial in Fragile X
We are pleased to share great news adapted from Neuren’s press release: Neuren’s phase 2 trial has successfully established proof of concept and provides a strong rationale for Neuren to move forward with developing trofinetide for Fragile X syndrome. In this initial small trial with a relatively short treatment period, trofinetide was very well tolerated, with the high dose (70 mg/kg twice daily) demonstrating a consistent pattern of clinical improvement, observed in both clinician and caregiver assessments.