Fragile X Research Funding of $1 Million Offered for 2021
FRAXA plans to fund $1 million for a generous number of Fragile X research grants and fellowships in 2021. Our mission is to find specific treatments and ultimately a cure for Fragile X syndrome. We aim to bring practical treatment into current medical practice as quickly as possible; we prioritize projects that have a clear practical application and the results of which will be shared in a timely fashion.
Integrating Human and Mouse Studies in Fragile X Syndrome – an NIH Center Approach
Presentations by Craig Erickson, Ernest Pedapati, Devin Binder, and Kimberly Huber about their research on Fragile X as part of their NIH Center of Excellence.
Developing Arbaclofen for Fragile X – Dr. Mark Bear 1:1 with FRAXA
Seven years ago, arbaclofen (STX209) was pulled from development, disappointing families around the US. Now MIT professor and FRAXA Investigator Dr. Mark Bear has founded Allos Pharma to bring it back. Dr. Bear sat down with FRAXA co-founder Katie Clapp to share the story and next steps.
Bryostatin-1 in Long-term Use Seen to Arrest Fragile X Symptoms in Mouse Model
Long-term, but not short-term, treatment with bryostatin-1 — Neurotrope’s lead investigational therapy — arrested such behavioral and cognitive symptoms as hyperactivity, difficulties with daily life activities, and learning and memory deficits in a mouse model of Fragile X syndrome.
Allos Pharma Revives Arbaclofen After 7 Years: New Hope for Fragile X Syndrome
Experience the revival of arbaclofen as Allos Pharma Inc launches a new development program, providing renewed hope for the Fragile X community. Discover the impact of this experimental drug and the determination of those who never gave up.
Positive Results Reported in Phase II Fragile X Clinical Trial of PDE4D Inhibitor Zatolmilast from Tetra Therapeutics
Today, Tetra Therapeutics announces the first unequivocally positive phase 2 clinical trial in Fragile X syndrome, press release below. The results do not depend on carving out a subset of patients or post hoc analysis.
Overcoming the Placebo Effect in Fragile X Clinical Trials
In a placebo-controlled clinical trial, some participants are given an experimental medication, while others are given a placebo. Participants do not know whether they are taking medicine or placebo. In theory, this can allow researchers to rule out the placebo effect by comparing outcomes among the two groups. But, per Wexler (2020) “having a strong placebo effect can obscure any real effect of the therapy being investigated”.
Zygel May Improve Behavior in Children With Severe Fragile X, Trial Data Suggest
Zynerba presented clinical trial results for Zygel at a recent neurology conference. Zygel, an experimental cannabidiol (CBD) gel, may reduce behavioral abnormalities in children with Fragile X syndrome who have more severe disease.
Healx Drug Repurposing Programme for Fragile X Syndrome
David Brown, MD, PhD, Ivan Angulo-Herrera, PhD and Anthony Hall of Healx present about the Drug Repurposing Programme for Fragile X syndrome.
Yale Researcher Dr. Elizabeth Jonas 1:1 with FRAXA
We talk with Dr. Elizabeth Jonas, Professor of Internal Medicine and Neurology at Yale School of Medicine, about her new research suggesting that leaky mitochondria cause some symptoms of Fragile X syndrome. Leaky membranes may also be involved in Parkinson’s Disease and other diseases.
Aripiprazole (Abilify) in the Treatment of People with Fragile X: An Anecdotal Account
The aim of this article is to discuss the use of Abilify (generic name: aripiprazole) as a treatment for people with Fragile X syndrome (FXS). As an “off-label” prescription, Abilify targets behaviors such as irritability, aggression, self-injury and severe tantrums.
Brain Organoids and Therapeutic Development for Fragile X and Other Rare Diseases
This is the first in a series of webinars focused on current topics in Fragile X research. In this webinar we hear from Alysson R. Muotri, PhD, Professor at University of California San Diego Stem Cell Programand Fabio C. Tucci, PhD, Chief Operating Officer and co-founder at Epigen Biosciences, Inc.
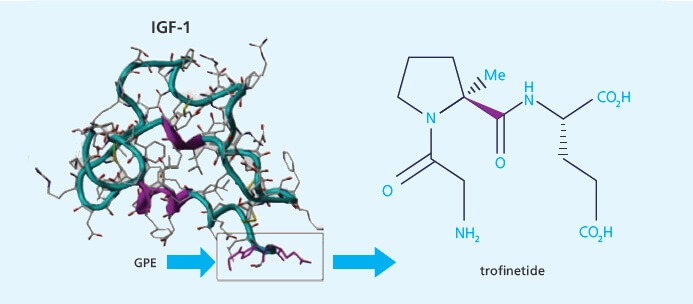
Trofinetide Clinical Trial Results Published
New Zealand-Based Biotech Neuren Pharmaceuticals Has Published Successful Phase 2 Fragile X Clinical Trial. Trofinetide, given to adolescent and adult males with Fragile X syndrome, was shown to be generally safe and was well-tolerated. It also showed preliminary evidence of efficacy. This trial validated a new design which can be used in future trials.
Scientists Find a New Way to Reverse Symptoms of Fragile X
FRAXA Investigator and MIT Professor Mark Bear and his colleagues have identified a valuable new target for Fragile X therapeutics: GSK3 alpha. Several FRAXA research teams previously identified GSK3 beta as a treatment target for Fragile X. The catch is that, so far, GSK3 beta inhibitors have proven too toxic for regular use. Dr. Bear’s new discovery opens up the possibility of developing more selective compounds with less toxicity and fewer side effects. Interestingly, lithium inhibits both GSK3 versions – alpha and beta.
Companies Move to Advance Potential Cognitive Treatment for Fragile X
Tetra Therapeutics and Shionogi announced plans to expand their partnership supporting BPN14770, a treatment candidate for disorders marked by cognitive and memory deficits, including Fragile X syndrome and Alzheimer’s disease. The agreement builds on an earlier collaboration between the two companies, and aims to further accelerate BPN14770’s development and potential marketing. It is currently in clinical testing in both Fragile X and Alzheimer’s patients.
Ketogenic Diet Eases Symptoms in Fragile X Male Mice
Westmark Lab is studying sleep patterns in Fragile X mice, working with UW-Madison to develop tools that speed EEG data analysis
Drug Repurposing for Rare Disease and the Future of Health – The Genetics Podcast
In this double-bill episode of The Genetics Podcast, Dr. Patrick Short talks to two key rare disease researchers in the field: Dr. Bruce Bloom, CCO of Healx, and Dr. Mike Tranfaglia, CSO of FRAXA. Both draw on their wide-ranging personal and professional experiences to discuss the successes and opportunities of drug repurposing, the power of using machine learning, and the work they’ve been doing to aid in finding effective treatments for Fragile X.
Less Active Immune System Evident in Fragile X Patients, Study Suggests
People with Fragile X syndrome are more likely to develop infections, but are less susceptible to autoimmune disorders than the overall population, a new study found. Taken together, this suggests that the immune system is underactive in this patient population. The study, titled, “The phenotypical implications of immune dysregulation in Fragile X syndrome,” was published in the European Journal of Neurology.
Results Reported: Using EEG Responses to Sound for Fragile X Drug Discovery
Jonathan Lovelace, a FRAXA funded Postdoc at UC Riverside, has made some exciting EEG findings over the past few years studying auditory hypersensitivity in mice and therapeutic drug treatments. A big obstacle in FXS research has been establishing reliable, unbiased, and translation relevant biomarkers that can be used to determine the effectiveness of therapies. One of the most important discoveries they have made is the striking similarity in EEG biomarkers between mice and humans.
Should You Participate in a Fragile X Clinical Trial?
A Fragile X clinical trial of a new PDE4D allosteric inhibitor from Tetra Therapeutics is nearly complete. Right now there are 3 remaining spots open to males 18-45 years of age with Fragile X syndrome. Dr. Elizabeth Berry-Kravis at the Rush University Medical Center in Chicago is leading this trial. The drug being studied has a unique mechanism of action that might improve cognitive and memory function.
New Partners Aim to Accelerate the Discovery and Repurposing of Medicines for Rare Neurological Diseases
First Healx secured $56M in new financing to launch a global Rare Treatment Accelerator program to tackle Fragile X syndrome and 39 other rare diseases. Now they have built a partnership with Boehringer Ingelheim worth millions. It all started with a small FRAXA grant to Healx to repurpose available drugs for Fragile X.
Ganaxolone Fragile X Clinical Trial Showed Disappointing Results
Ganaxolone, an experimental drug from Marinus Pharmaceuticals which targets GABA receptors, did not show promise for Fragile X syndrome in a clinical trial.
FXS Patients’ Social Deficits are Linked to Social Anxiety, Eye-tracking Study Says
Dr. Craig Erickson and colleagues at the University of Cincinnati used eye-tracking technology to understand sociability in Fragile X syndrome. This study affirms what so many parents, caretakers, and educators suspect: people with fragile X want to be social, and it is anxiety – not lack of interest – which usually hold them back. If anxiety could be reduced, more sociability would likely follow. Dr. Erickson is a Fragile X expert and FRAXA investigator who is currently conducting a Fragile X clinical trial of an investigational new drug.