Repurposing Available Drugs to Treat Fragile X Syndrome – FRAXA Initiatives
FRAXA Research Foundation was founded in 1994 to fund biomedical research aimed at finding a cure for Fragile X syndrome and, ultimately, autism. We prioritize translational research with the potential to lead to improved treatments for Fragile X in the near term.
Our early efforts involved supporting a great deal of basic neuroscience to understand the cause of Fragile X. By 1996, these efforts had already begun to yield results useful for drug repurposing. To date, FRAXA has funded well over $25 million in research, with over $3 million of that for repurposing existing drugs for Fragile X.
Here are some examples of FRAXA-funded work on repurposing available drugs for Fragile X syndrome:
Lithium
In the mid-1990s, the Greenough lab at the University of Illinois discovered that FMRP, the protein missing in Fragile X, is rapidly translated in dendrites in response to stimulation of glutamate receptors. FRAXA funded preclinical validation of this discovery in the Fragile X mouse model. This key result launched all the further studies.
1999: Darnell (Rockefeller) and Broadie (Vanderbilt) labs independently found that FMRP regulates MAP1b translation and GSK3 activity, suggesting lithium as a potential therapeutic. Lithium is an available drug which inhibits both GSK3 and MAP1b. FRAXA then funded the Bauchwitz lab at Columbia and the Jope lab at UAB to validate lithium and investigational GSK3 inhibitors in Fragile X animal models. FRAXA then funded a pilot clinical trial of lithium (Berry-Kravis at Rush University Medical Center) with good results; larger follow-up studies are still needed (NIH declined to fund these).
Baclofen
1998: The Toth lab (Cornell-Weill) described sensory hypersensitivity in Fragile X mice which can be treated with baclofen. FRAXA then partnered with Seaside Therapeutics for clinical trials of arbaclofen, a proprietary version of the available drug. Seaside went out of business, but many patients with Fragile X continue on baclofen today.
Minocycline
2007: The Ethell lab (UCR) found excessive activity of matrix metalloproteinases (MMP-9) in Fragile X, resulting in impaired synaptic maturation. The available drug minocycline, a known MMP-9 inhibitor, was shown to rescue this deficit. FRAXA organized and funded a clinical trial of minocycline, demonstrating excellent clinical response. Many Fragile X patients currently take minocycline. FRAXA went on to partner with Paratek to investigate other agents which can inhibit MMP-9 without antibiotic activity. Another clinical trial of minocycline by Dr. Randi Hagerman in 2013 also showed good results.
Lovastatin
2010: The Bear lab (MIT) showed that the available drug lovastatin can reduce mGluR signaling and reverse signs of Fragile X in animal models. FRAXA funded clinical trials of lovastatin in Quebec, with promising results. FRAXA is currently funding a follow-up combined study of lovastatin and minocycline in patients with Fragile X.
Green Tea Extract
2012: FRAXA funded preclinical studies in Fragile X mice and a double blind, randomized clinical trial in Fragile X patients using Mega Green Tea Extract in the Barcelona, Spain lab of Dr. Mara Dierssen. Dr. Dierssen has submitted a paper reporting results of this study, and we are awaiting results.
Metformin
2014: After multiple investigators (Vanderklish at Scripps, Sonenberg at McGill, Jongens and McBride at UPenn) discovered potential therapeutic effects of metformin in Fragile X animal models, FRAXA funded a study to validate metformin in live animal model at FRAXA-DVI (see below).
FRAXA's Global Repurposing Strategy
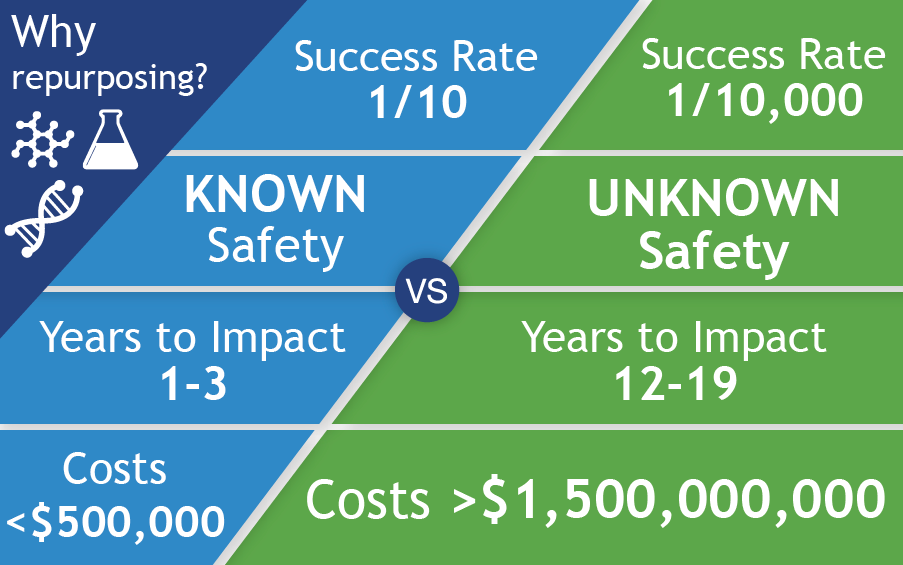
Because repurposing available drugs is so much faster and more cost-effective than advancing investigational drugs, we’ve launched additional projects with wide-ranging potential.
In 2012, FRAXA established the Drug Validation Initiative (FRAXA-DVI) in South America to conduct rapid validation of candidate drugs in Fragile X animal models. This has led to more than 30 currently active partnerships with pharmaceutical companies. FRAXA-DVI also allows for validation of available drugs emerging from studies by other labs without in vivo capability.
FRAXA is partnering with Healx (Cambridge, UK) to conduct repurposing studies based on transcriptional profiling. This project is expected to produce a ranked list of potentially useful drugs which we plan to further test at FRAXA-DVI. We are organizing clinical trials of the most promising available drugs – notably metformin – to assess these drugs and to gain a better understanding of biomarkers, outcome measures, and overall clinical trial design for Fragile X, autism, and related developmental disorders.
While there is still a lot more work to do before any of these strategies can be considered proven therapies for Fragile X, we are excited about the possibilities of repurposing existing drugs for Fragile X. We eagerly look forward to the results of these and future studies!
Written by
Michael Tranfaglia, MD
Medical Director, Treasurer, Co-Founder
Dr. Michael Tranfaglia is Medical Director and Chief Scientific Officer of FRAXA Research Foundation, coordinating the Foundation’s research strategy and working with university and industry scientists to develop new therapeutic agents for Fragile X. He has a BA in Biology from Harvard University and an MD from the University of North Carolina at Chapel Hill. His son Andy has Fragile X syndrome.

