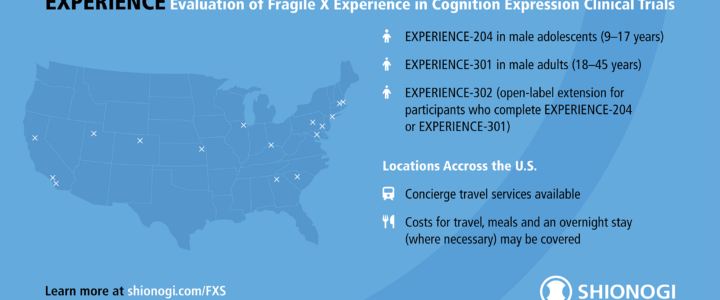
Shionogi’s EXPERIENCE clinical trials for Fragile X syndrome are nearing completion. Enrollment for the adult trial (EXPERIENCE-301) is now closed, while the adolescent trial (EXPERIENCE-204) is in its final phase. Learn more about the study, FRAXA’s role, and the open-label extension.
Read moreBerry-Kravis, Elizabeth
Elizabeth Berry-Kravis, MD and PhD, is the Co-Director of the Molecular Diagnostics Section of the Genetic Laboratory at Rush University. She studies genotype- and phenotype-related neurodegenerative and neurogenetic diseases. Dr. Berry-Kravis has a history of studying Fragile X and established the comprehensive Fragile X Clinic and Research Program at Rush University in 1992. She has won several awards, including the FRAXA Champion Award in 2011.
Innovative Breakthrough in Fragile X Treatment: The Promise of Antisense Oligonucleotide (ASO) Therapy

This changes everything! FRAXA funded research introduces Antisense Oligonucleotide (ASO) Therapy, redefining Fragile X syndrome treatment and understanding.
Read more10 Year Vision for Fragile X Research – Dr. Elizabeth Berry-Kravis & Dr. Patricia Cogram

In this video we hear from FRAXA Investigators Dr. Patricia Cogram, Professor at the University of Chile, and Dr. Elizabeth Berry-Kravis, Professor at Rush University Medical Center as they reflect on the progress that has been made and visualize what they see happening in the next 10 years for people living with Fragile X syndrome.
Read moreFragile X Clinical Trial of New PDE4D Inhibitor from Tetra

With a $200,043 grant from FRAXA Research Foundation, Dr. Elizabeth Berry-Kravis completed a successful Phase 2 clinical trial of a PDE4 inhibitor for adult men with Fragile X syndrome. This trial treated 30 males, 18-45 years of age with a new PDE4D allosteric inhibitor from Tetra Discovery Partners using a crossover design, so that everyone got active drug for part of the time and placebo for part of the time.
Read moreFX-Learn Clinical Trial for Children with Fragile X

Thirteen centers across the US enrolled children with Fragile X in a large-scale clinical trial of Novartis AFQ056. Dr. Elizabeth Berry-Kravis and colleagues aim to show that this targeted treatment — an mGluR5 blocker for Fragile X which failed in previous adult human trials — can be better evaluated by studying effects on learning in young children.
Read morePurposeful and FRAXA Partnership Leads to Clinical Trial

Can a combination of drugs make a meaningful difference for people with Fragile X? A new clinical trial is going to find out. 15-20 adult men with Fragile X will be included in this trial to test the effects of an available drug and a nutritional supplement taken together.
Read moreTetra’s Fragile X Clinical Trial – The Most Successful So Far

Dr. Mark Gurney, CEO of Tetra Therapeutics, discusses how one of the earliest clues to the biology of Fragile X led to the most successful Fragile X clinical trial to date. FRAXA and Tetra began working together after a key FRAXA-funded study caught the attention of Dr. Gurney. Through the FRAXA Drug Validation Initiative, Dr. Patricia Cogram was able to conduct preclinical validation experiments with Tetra’s lead compound in record time, paving the way for clinical trials.
Read moreAlternative Splicing in White Blood Cells: A Biomarker for Fragile X Syndrome

Explore groundbreaking research by the University of Massachusetts Medical School and Rush University Medical Center on alternative splicing in white blood cells as a biomarker for Fragile X syndrome, paving the way for personalized treatment optimization through a non-invasive blood test.
Read more2021 FRAXA Awards – Recognizing Perseverance and Dedication

In conjunction with World Fragile X Day 2021, FRAXA Research Foundation is proud to recognize its annual award recipients. This year’s recipients exemplify the perseverance and dedication that has made FRAXA a global leader in Fragile X research for nearly 30 years. We are fortunate to partner with these individuals on research, community support and awareness efforts.
Read moreClinical Trials and Cyclic AMP in Fragile X Syndrome: A Life Journey

In November 2020, a phase II clinical trial reported extremely successful results. This clinical trial of a PDE4D inhibitor from Tetra Pharmaceuticals was conducted by Dr. Elizabeth Berry-Kravis at Rush University Medical Center and funded by FRAXA Research Foundation. In this Simons Foundation lecture, Elizabeth Berry-Kravis traces 30 years of Fragile X research, from identifying its cause, through finding dozens of treatment targets, through a series of disappointing clinical trials.
Read morePositive Results Reported in Phase II Fragile X Clinical Trial of PDE4D Inhibitor Zatolmilast from Tetra Therapeutics

Today, Tetra Therapeutics announces the first unequivocally positive phase 2 clinical trial in Fragile X syndrome, press release below. The results do not depend on carving out a subset of patients or post hoc analysis.
Read moreTetra Discovery Partners Initiates Phase 2 Trial of BPN14770 in Fragile X Syndrome

This 2-Period Crossover Study of BPN14770 is accepting adults males with Fragile X syndrome at Rush University Medical Center in Chicago. Principal Investigator of the study is Elizabeth Berry-Kravis, MD, PhD.
A selective inhibitor of the phosphodiesterase type-4D (PDE4D), BPN14770 has shown the ability to improve the quality of connections between neurons and to improve multiple behavioral outcomes in the Fragile X mouse model.
New Fragile X Clinical Trial for Children Launching in June 2017

Rush University Medical Center Professor Elizabeth M. Berry-Kravis, MD, PhD, has launched and is recruiting participants for a large-scale clinical trial to study effects of AFQ056, an mGluR5 blocker, on learning in young children.
Read moreClinical Trials Outcome Measures

With $281,824 in funding from FRAXA Research Foundation from 2002-2011, Dr. Berry-Kravis at the Rush University Medical Center attempted to validate a new automated video tracking system for quantifying physical activity as an outcome measure for Fragile X clinical trials.
Read morePilot Clinical Trial of Lithium in Fragile X Shows Promising Results

With a $65,000 grant from FRAXA Research Foundation in 2005, Dr. Berry-Kravis at the Rush University Medical Center conducted a pilot clinical trial of lithium in 15 patients with Fragile X syndrome. Results published.
Read more