Learn how Dr. Marine Anais Krzisch’s $35K FRAXA and ASF-funded project uses human iPSC microglia models to uncover pathways for Fragile X syndrome treatment.
Read moreautism
Modeling R-Loop Therapy for Fragile X Syndrome in Patient-Derived Brain Organoids

Fragile X syndrome researchers model R-loop therapy in patient-derived brain organoids to restore FMR1, accelerating a curative approach supported by FRAXA.
Read moreFragile X and PDE Inhibitors: A Promising Path Forward for Brain Disorders

Fragile X syndrome research shows PDE inhibitors may improve brain function and behavior, with promise for related neurodevelopmental disorders.
Read moreShionogi’s EXPERIENCE Phase 3 Clinical Trial of Zatolmilast in Fragile X Syndrome
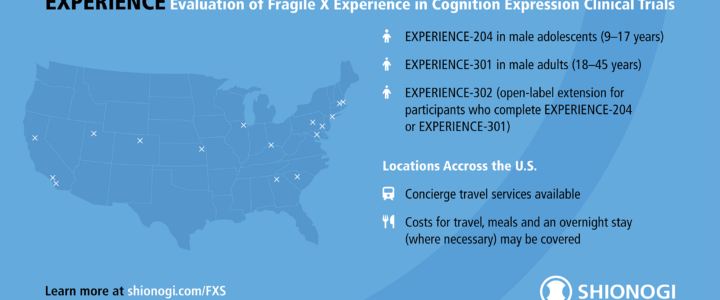
Shionogi’s EXPERIENCE clinical trials for Fragile X syndrome are nearing completion. Enrollment for the adult trial (EXPERIENCE-301) is now closed, while the adolescent trial (EXPERIENCE-204) is in its final phase. Learn more about the study, FRAXA’s role, and the open-label extension.
Read moreUrgent Action Needed: Help Secure NIH Funding for Fragile X Research

NIH funding delays are threatening Fragile X research, putting critical studies and future treatments at risk. FRAXA Research Foundation and NFXF are urging Congress and the NIH to act now. Learn how you can help secure funding for Fragile X research.
Read moreFRAXA-Funded Research Explores ISRIB as a Potential Treatment for Fragile X

ISRIB for Fragile X syndrome is being studied as a potential treatment to restore brain function and social behavior. Researchers investigate its effects.
Read moreCallum Cup VIII Scores $19,400 for Fragile X Research – A Milestone Event

Callum Cup VIII, Millburn FC’s annual charity match, scored big with $19,400 raised for Fragile X research, bringing total funds to $148,000. Discover how soccer unites for a cause.
Read moreHelp Rowan Thrive: Support Fragile X Research

Join the Gale family in supporting Fragile X research through FRAXA. Help Rowan and others thrive by funding life-changing treatments and advancing curative therapies.
Read moreSupport the Stevenson Family Campaign

The march of time renews the commitment we made to a special needs community 25 years ago. We vowed to dream big and never give up until there were effective treatments available and eventually a cure for Fragile X syndrome, the most commonly inherited cause of intellectual disabilities and autism.
Read moreNPR Spotlights Zatolmilast: A Potential Breakthrough for Fragile X Syndrome

NPR spotlights zatolmilast, a promising drug offering new hope for individuals with Fragile X syndrome. Families report life-changing improvements in anxiety, communication, and independence.
Read moreMarvel Biosciences Partners with FRAXA to Test MB204 for Fragile X Syndrome

Discover how Marvel Biosciences and FRAXA Research Foundation are collaborating to test MB204, a promising new treatment for Fragile X syndrome, building on groundbreaking adenosine receptor research.
Read moreFragile X Treatment Target Emerges from Neurolixis & FRAXA Collaboration
A new treatment target for Fragile X syndrome has emerged from multiple research labs and a pharmaceutical startup. Neurolixis, a FRAXA pharma partner, has announced a new Fragile X development program.
Read moreBK Channel Openers: A New Drug for Fragile X Is Ready for Clinical Trials
Discover the promising new BK channel opener, SPG601, now entering clinical trials for Fragile X syndrome. Learn about its potential to restore synaptic function and address core symptoms.
Read moreRenewed Hope: Navigating Towards a Cure for Fragile X Syndrome

Discover how Dr. Peter Todd’s latest Fragile X Syndrome research offers hope for advanced treatments and a possible cure, marking a new era in FXS therapy.
Read morePharmacologically Activating mGluR7 as a Novel Therapy for Fragile X Syndrome
Join Dr. Tsai and Dr. Kumar on a journey into novel treatment avenues for Fragile X syndrome. Learn how activating mGluR7 could be a game-changer, opening up uncharted therapeutic territory.
Read moreCallum Cup’s Big Win: Millburn Charity Soccer Raises $26K for Fragile X Research

Discover the inspiring story of the Callum Cup in Millburn, NJ – a charity soccer match that raised over $26,000 for Fragile X research. Join us in celebrating community spirit and a commitment to a vital cause.
Read more$100,000 Matching Challenge From The Robert & Ardis James Foundation

We are thrilled to announce FRAXA Research Foundation’s most significant and unique matching challenge of the year, thanks to the Robert & Ardis James Foundation. This challenge will help us bring top new talent to Fragile X research.
Read moreSomatosensory Processing as a Therapeutic Target for Fragile X Syndrome
Awarded a FRAXA Research grant, Dr. Andrew Stanfield, Dr. Leena E. Williams, and Dr. Damien Wright are set to explore somatosensory processing (sense of touch) in Fragile X syndrome at the University of Edinburgh. Their aim? A noninvasive touch test that could set the stage for future clinical trials in FXS.
Read moreAntisense Oligonucleotides (ASOs) to restore FMRP in Human Fragile X Cerebral Organoids

Explore Dr. Richter’s encouraging results with ASOs for Fragile X syndrome. A $100,000 grant now fuels pivotal studies needed to advance toward ASO therapy.
Read moreFRAXA Research Foundation Partners with Autism BrainNet
Discover how FRAXA Research Foundation’s collaboration with Autism BrainNet accelerates Fragile X syndrome research by collecting vital postmortem brain tissue. Dive into the significance of brain studies for deeper insights and potential therapeutic interventions.
Read moreASOs and Fragile X: Addressing the Most Asked Questions

Explore the potential of ASOs in treating Fragile X syndrome & FXTAS. Dive into a comprehensive Q&A addressing key questions and breakthrough findings.
Read moreIn Memory of Garret

Honor Garret Peacock Volker’s extraordinary life by supporting FRAXA’s mission to find a cure for Fragile X syndrome. Your donation can make a difference in the lives of those affected.
Read morePioneering Community-Based Drug Development for Fragile X Syndrome

Discover how FRAXA leverages Community-Based Drug Development to create impactful therapies for Fragile X syndrome. Join us as we reshape the future of rare disease treatment.
Read moreAllos Pharma Advances Phase 3 Clinical Trial Design for Potential Fragile X Syndrome Treatment, Arbaclofen

Discover Allos Pharma’s advancements in a pivotal Phase 3 trial for Fragile X syndrome treatment, Arbaclofen. Learn how their FDA-informed trial design might finally bring hope to the Fragile X community.
Read moreBreakthrough Discoveries in Fragile X Research: Insights from Special Banbury Meeting on Curative Therapies

Explore the latest breakthroughs in Fragile X research unveiled at the recent Banbury Meeting. Discover novel strategies, from gene therapy to protein replacement, that bring hope for curative therapies.
Read more
