Clinical Trials in Fragile X Syndrome
To all who have loved ones impacted by Fragile X syndrome, we sincerely thank you for your interest in clinical trials. These trials are beacons of hope during challenging times, and your curiosity and engagement are instrumental in pushing this important research forward.
As a further resource, we offer an enlightening video discussion featuring Holly Roos and Katie Clapp, both mothers to young adults with Fragile X and key figures in FRAXA. Their personal experiences provide unique and heartfelt perspectives on the journey through Fragile X clinical trials.
We understand that you might have questions about what participating in a clinical trial entails. Each study is supervised by a medical professional specializing in Fragile X syndrome. Participation is free, with no need to inform your insurance provider and the freedom to withdraw at any time. Typically, travel expenses are covered.
It's important to know that the FDA usually mandates two successful large-scale clinical trials before considering the approval of a new treatment, however for rare conditions like Fragile X syndrome, one trial with strong results may be sufficient. Upon successful trials and subsequent FDA approval, new drugs become accessible to others diagnosed with Fragile X syndrome, hopefully improving the quality of life for many individuals and families.
Ongoing Fragile X Studies Accepting Participants
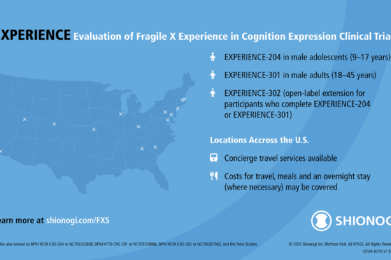
Recruiting: Mirum Launches Clinical Trial of MRM-3379 for Fragile X Syndrome
Mirum’s MRM-3379 Phase 2 trial builds on FRAXA-funded PDE4D research and is now enrolling males ages 13–45 with Fragile X syndrome. View eligibility and study sites.
Additional Fragile X Clinical Trials
Please visit clinicaltrials.gov for a complete listing of Fragile X syndrome clinical trials.