Explore groundbreaking research on the potential of Cannabidiol (CBD) in modulating the endocannabinoid system for Fragile X syndrome therapy. Discover how CBD could change the natural course of Fragile X.
Read moreRoche
Lovamix: Clinical Trial of Combined Treatment of Minocycline and Lovastatin in Fragile X Syndrome

With a $66,714 grant from the FRAXA Research Foundation awarded over 2015-2017, Dr. Francois Corbin at the Universite of Sherbrooke will test the safety and synergistic effects of lovastatin and minocycline in patients with Fragile X syndrome.
Read moreCorrecting the Brain’s Emotional Memory Center
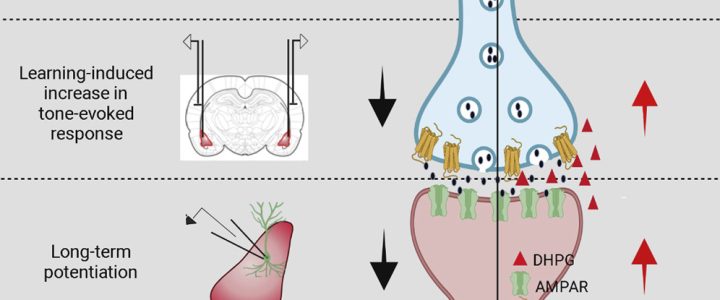
Cell Reports has published the results of the study “Correction of Amygdalar Dysfunction in Rat Model of Fragile X Syndrome” by FRAXA Investigator Dr. Sumatra Chattarji and his team at the National Centre for Biological Sciences in Bangalore, India. The researchers investigated the synaptic basis of deficient conditioned fear and its reversal in Fragile X syndrome rats.
Read moreDrug Tolerance in MGluR5 Clinical Trials – Dr Patrick McCamphill 1:1 with FRAXA

We have long suspected that the clinical trials of mGluR5 blockers from Novartis and Roche failed because the drug triggered tolerance, losing effect over time. With a $90,000 grant from FRAXA, Dr. Patrick McCamphill, a Postdoctoral Fellow in the MIT lab of Dr. Mark Bear, is investigating. He does indeed find tolerance, and now he is looking for ways to overcome it.
Read moreBeneath the Surface of Fragile X Syndrome: Study Sheds Light on What’s Happening in Nerve Cells
This FRAXA-funded project has turned up some surprising results. At first, it might seem Kurosaki and Maquat have found yet another cellular process which is malfunctioning in Fragile X. But this finding is intimately related to previous findings of abnormal protein synthesis and misregulated transcription in Fragile X. FMRP (the protein lacking in Fragile X syndrome) is involved in chaperoning messenger RNAs within cells to active sites, and in controlling their translation into many different proteins. Some of these proteins are transcription factors, which feed back to the nucleus to control gene expression.
Read moreHealx Drug Repurposing Programme for Fragile X Syndrome

David Brown, MD, PhD, Ivan Angulo-Herrera, PhD and Anthony Hall of Healx present about the Drug Repurposing Programme for Fragile X syndrome.
Read moreKetogenic Diet Eases Symptoms in Fragile X Male Mice

The Westmark laboratory continues to study sleep and rest-activity cycles in Fragile X mice as a potential outcome measure that correlates between preclinical and clinical research. The analysis of sleep EEG in the mice has proven more labor intensive than they anticipated, but the team is collaborating with Dr. Rama Maganti’s laboratory at UW-Madison on the development of computer scrips to speed up the analysis.
Read moreFRAXA Biotech Games, the Beginning of Something Great

On September 20, 2018, FRAXA Research Foundation held the First Annual FRAXA Biotech Games™. The event was a “friendly” competition between greater Boston biotech companies and affiliated industry partners and vendors in a series of fun backyard lawn games. 42 teams of 4 players each played cornhole, KanJam, ladder golf and bucketball. Our goal was to establish an annual event in Cambridge, MA, that would unite the biotech community for an afternoon of fun competition, and raise money for biomedical research. We look forward to the upcoming Biotech Games!
Read moreHow Promising is CRISPR for Fragile X?

Peter Todd, MD, PhD, Assistant Professor in the Department of Neurology in the University of Michigan Medical School, was awarded a FRAXA Research Grant for gene reactivation with the use of CRISPR. In this interview he tells us about CRISPR in Fragile X research, how realistic is it that it could turn the Fragile X gene back on, and if it can really cure Fragile X.
Read morePharmacological Tolerance in the Treatment of Fragile X Syndrome

With a $90,000 grant from FRAXA Research Foundation over 2018-2019, Dr. Patrick McCamphill, postdoctoral fellow in Dr. Mark Bear’s lab at Massachusetts Institute of Technology (MIT), is investigating drug tolerance to mGluR5 antagonists, arbaclofen, and other potential Fragile X treatments. He is also exploring ways to overcome it.
Read moreNewly Discovered Regulatory Pathways in Fragile X

Studies at Yale University and elsewhere are showing that FMRP plays a significant role in the regulation of potassium channels. Looking forward, potassium channel opener drugs could rescue some symptoms of Fragile X in humans.
Read moreBrain Imbalance Target of Dr. Erickson’s New Clinical Trial

According to Dr. Erickson, AZD7325 is a drug that selectively boosts GABA neurotransmission in the brain. GABA is the primary neurochemical in the brain that blocks brain activation. GABA activity is in balance in the brain with Glutamate activity, which is the primary neurochemical that causes brain activation. In Fragile X, GABA activity is insufficient and glutamate activity is excessive, likely causing brain activity to be out of balance. AZD7325 attempts to correct parts of this imbalance by boosting the insufficient GABA activity in the brains of people with Fragile X.
Read moreMechanisms of Tolerance to Chronic mGluR5 Inhibition

Over the past few years, both Novartis and Roche sponsored large-scale clinical trials of metabotropic glutamate receptor 5 (mGlu5) negative allosteric modulators (NAMs) to treat Fragile X syndrome (FXS). With a $90,000 grant from FRAXA Research Foundation in 2015-2017, Dr. Mark Bear’s team will explore if mGlu5 NAMs dosed chronically causes tolerance, and if so, how it develops and to probe new avenues to prevent or circumvent it.
Read moreMetformin, Diabetes Drug, Potential Fragile X Treatment

“We treated mice with metformin and corrected all the core Fragile X deficits. We are optimistic about using metformin in human clinical trials. This is a generic drug with few side effects” says Nahum Sonenberg, PhD, James McGill Professor, Department of Biochemistry, McGill Cancer Center, McGill University.
Read moreFragile X Nervous (System) Breakdown

“The occurrence and development of events by chance in a happy or beneficial way.” That’s how Lynne E. Maquat, PhD, describes the process of how her research extended to Fragile X syndrome to better understand it and ultimately find advanced treatments.
Read moreRepurposing Drugs to Dampen Hyperactive Nonsense-Mediated Decay in Fragile X Syndrome

With a $90,000 grant from the FRAXA Research Foundation, Dr. Lynne Maquat and Dr. Tatsuaki Kurosaki will investigate nonsense-mediated mRNA decay (NMD) in Fragile X. NMD is a “housekeeping” process that cells use to prevent faulty proteins from being made. But there is too much of it in Fragile X syndrome. There are already available drugs that suppress NMD – including caffeine.
Read moreDouble Down: Fragile X Clinical Trial Combines Two Available Drugs
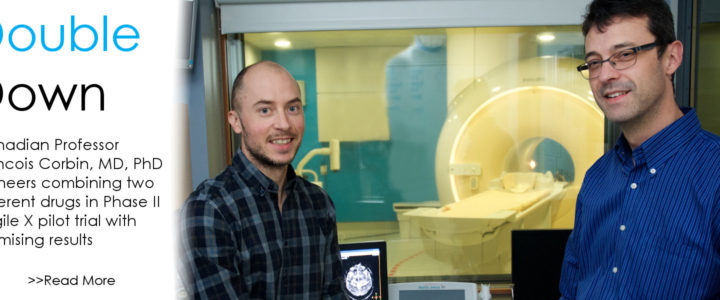
If all the science world’s a stage, Fragile X researchers are more than merely players. They are center stage. So believes Francois Corbin, MD, PhD, professor, Université de Sherbrooke, Canada, who directs the university’s Fragile X Clinic. Corbin, who has received more than $100,000 in FRAXA support since 2012, is leading a pilot randomized Phase II trial, exploring the tolerability and the synergistic effect of a combined therapy.
Read moreBoston Bruins Grant Funds New Fragile X Research

Bruins Foundation Executive Director Bob Sweeney pledging a $90,000 donation to FRAXA Research today at Shared Living Collaborative’s Gateway Farm in Merrimac, MA. The award will enable the organization to fund an entirely new research project aimed at developing new treatments for Fragile X, a genetic syndrome that is the most common inherited cause of autism.
Read moreRoche reports clinical trial negative results

Roche has shared the sad news that their clinical trials in Fragile X have been unsuccessful. They will host a Webcast on Thursday, September 18, from 12:30pm – 1:30pm (EDT) to explain the results. For details and dial-in information please see this letter from Luca Santarelli, the
Read moreFragile X Clinical Trial: Novartis Trial Results Are In, and They’re Not Pretty
This year’s Gordon Conference just finished, and Novartis presented their results for the first time (though advisors and advocates had been given a private peak months ago.) To say that the trial results for AFQ056 were disappointing would be the understatement of the century!
Read moreClinical Trials FAQ ← Frequently Asked Questions
Question: How Do Families Decide Which Trial is Best for Them? Answer: Each of the trials has different requirements for joining, so many – if not most – people will only be eligible for one trial after screening. The best way to approach this is to call the clinic contact closest to your area and discuss this with him/her. Age, weight, current medications, behavior, and IQ are all factors.
Read moreA Metabolomic Drug Efficacy Index to Test Treatments in the Fragile X Mouse

Dr. Davidovic has been examining changes in metabolism in various brain regions that are affected in Fragile X patients. She has defined a brain-specific metabolic signature of FXS and is testing treatment strategies to restore normal levels of these metabolites.
Read moreCompound that Inhibits mGluR5 Corrects Signs of Fragile X in Adult Mice
A study finds that a new compound reverses many of the major symptoms associated with Fragile X syndrome (FXS). The paper is published in the April 12 issue of the journal Neuron, describing the exciting observation that the FXS correction can occur in adult mice, after the symptoms of the condition have already been established. Previous research has suggested that inhibition of mGlu5, a subtype of receptor for the excitatory neurotransmitter glutamate, may ameliorate many of the major symptoms of the disease. This study, a collaboration between a group at Roche in Switzerland, led by Dr. Lothar Lindemann, and Dr. Mark Bear’s MIT lab, used an mGlu5 inhibitor called CTEP to examine whether inhibition of mGlu5 could reverse FXS symptoms.
Read more3 Researchers Honored at FRAXA Investigators Meeting
Over 150 scientists from around the globe gathered in Durham, New Hampshire, for FRAXA Research Foundation’s Investigators Meeting on September 21-24, 2008. They came from Australia, Canada, India, Turkey, the U.S., and eight European countries. Their common goal: “to share, collaborate and publish,” in the words of FRAXA’s Medical Director, Michael Tranfaglia, MD, to find effective treatments and a cure for Fragile X, the foremost inherited cause of mental retardation and autism. Most of the attendees were university-based professors, postdoctoral fellows, and graduate students who have FRAXA research grants. Also participating in the meeting were scientists from the National Institutes of Health (NIMH, NICHD, and NINDS), Neuropharm Group PLC, Hoffman LaRoche Inc., GlaxoSmithKline, Indevus, and Seaside Therapeutics, as well as 20 parents of Fragile X children.
Read more
