Nospharma Announces Partnership with FRAXA to Test NOS-01 in Pre-Clinical Models of Fragile X
NOS-01 enters the FRAXA Drug Validation Initiative (FRAXA-DVI) for Fragile X mouse-model testing, generating data to guide development decisions.
FRAXA Drug Validation Initiative (FRAXA-DVI)
The FRAXA Drug Validation Initiative (FRAXA-DVI) provides speedy, cost-effective, objective preclinical testing to validate investigational and repurposed compounds for Fragile X.
BK Channel Openers: From FRAXA Seed Funding to Big Pharma Investment and Trials
See how FRAXA’s early grants propelled Fragile X research from lab discoveries to industry momentum, with partners advancing therapies into trials.
GEXVal and FRAXA Collaborate to Advance Fragile X Research with Phase 2a Trial
GEXVal and FRAXA collaborate to advance Fragile X research with the Phase 2a trial of GXV-001, supported by AMED’s funding program.
BK Channel Openers: A New Hope for Fragile X Treatment – Insights from Kaerus Bioscience CEO Robert Ring
Kaerus Bioscience’s BK channel openers for Fragile X syndrome are advancing through Phase 1 trials, offering hope for new treatments with FRAXA’s continued support.
Marvel Biosciences Partners with FRAXA to Test MB204 for Fragile X Syndrome
Marvel Biosciences and FRAXA Research Foundation are collaborating to test MB204, a promising new treatment for Fragile X which targets adenosine receptors.
Fragile X Treatment Target Emerges from Neurolixis & FRAXA Collaboration
FRAXA pharma partner Neurolixis has filed patents for treatment of Fragile X with a highly selective new drug, NLX-101, which targets the serotonin receptor 5-HT1A.
BK Channel Openers: A New Drug for Fragile X Is Ready for Clinical Trials
A promising new BK channel opener, SPG601 from Spinogenix, is entering clinical trials for Fragile X syndrome. Learn about its potential to restore synaptic function and address core symptoms.
Inside the FRAXA Drug Validation Initiative: Advancing Fragile X Treatments
FRAXA-DVI is revolutionizing Fragile X syndrome research, providing efficient, comprehensive and objective preclinical testing of potential treatments.
Two-Med Combo Normalized Behavior, Improved Memory in Fragile X Mice
Treating Fragile X might require a combination of drugs. FRAXA-DVI tested ibudilast and gaboxadol in Fragile X mice. Together they rescued a wide array of symptoms.
Coming Together for Rare Disease Day 2023
Today we mark Rare Disease Day. FRAXA is committed to advancing research on Fragile X, one of the most common rare diseases worldwide.
Fragile X Clinical Trial of New PDE4D Inhibitor from Tetra
A $200K FRAXA grant enabled a successful Phase 2 trial of a PDE4D inhibitor for adult men with Fragile X, showing strong cognitive gains without side effects or tolerance.
New Fragile X Clinical Trial Announced by Healx
Healx’s AI-driven approach makes finding the right combination therapies more efficient, cost-effective, and rapidly ready for testing at FRAXA-DVI. It was this process that has brought Healx to its recent announcement sharing that it has received Investigational New Drug (IND) approval from the US Food and Drug Administration (FDA) for the Phase 2a clinical study of HLX-0201 (sulindac, an FDA-approved drug).
Purposeful and FRAXA Partnership Leads to Clinical Trial
AI and FRAXA-DVI identified a drug + supplement combo that reversed all Fragile X symptoms in mice. A clinical trial tested this in adults with Fragile X.
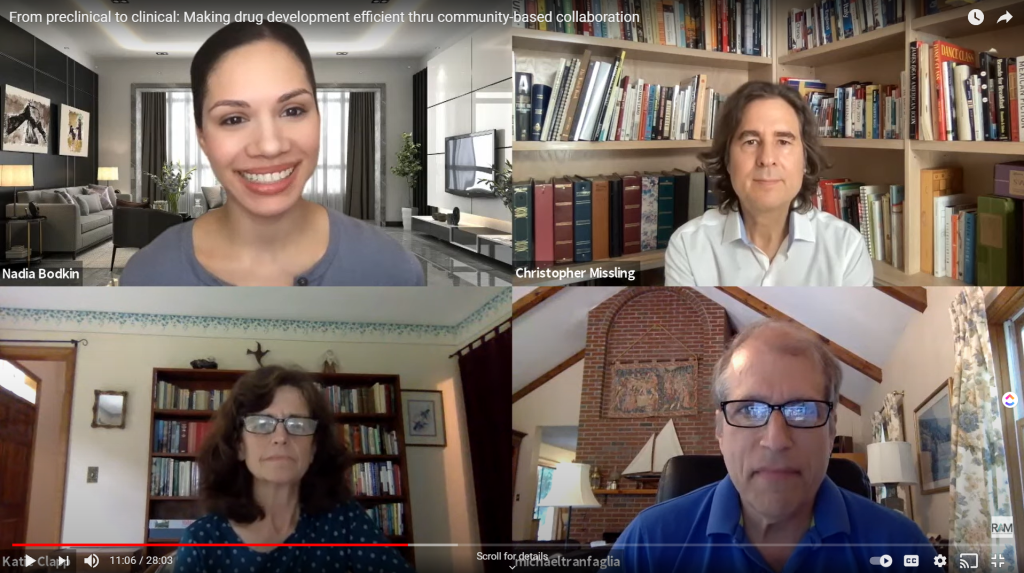
Making Drug Development Efficient Through Community-Based Collaboration
FRAXA’s partnership with Anavex shows how early collaboration between patient advocates and pharma can accelerate drug development for Fragile X and rare diseases.
Tetra’s Fragile X Clinical Trial – The Most Successful So Far
Dr. Mark Gurney, CEO of Tetra Therapeutics, discusses how one of the earliest clues to the biology of Fragile X led to the most successful Fragile X clinical trial to date. FRAXA and Tetra began working together after a key FRAXA-funded study caught the attention of Dr. Gurney. Through the FRAXA Drug Validation Initiative, Dr. Patricia Cogram was able to conduct preclinical validation experiments with Tetra’s lead compound in record time, paving the way for clinical trials.
Promising Results of Preclinical Study of ANAVEX®2-73
We are excited to share that Anavex Life Sciences announced today that preclinical data of the ANAVEX®2-73 (blarcamesine) study in Fragile X syndrome were published in the peer-reviewed journal, Scientific Reports.
Synaptogenix Announced Intention to Launch a Fragile X Clinical Trial with Bryostatin
Bryostatin research has advanced from mouse models to human trials. Synaptogenix and Nemours make plans to test this potential treatment in Fragile X clinical trials.
Tetra Releases Full Results of FRAXA-Funded Clinical Trial of PDE4D Inhibitor
Today, Tetra Therapeutics published the full results of its PDE4D trial published the full results to their announcement. Now having reviewed the full results, FRAXA can confidently say that the PDE4D drug trial gives hope to patients and families that Fragile X Syndrome is a treatable disorder, and this particular drug can improve intellectual disability.
Bryostatin-1 in Long-term Use Seen to Arrest Fragile X Symptoms in Mouse Model
Long-term, but not short-term, treatment with bryostatin-1 — Neurotrope’s lead investigational therapy — arrested such behavioral and cognitive symptoms as hyperactivity, difficulties with daily life activities, and learning and memory deficits in a mouse model of Fragile X syndrome.
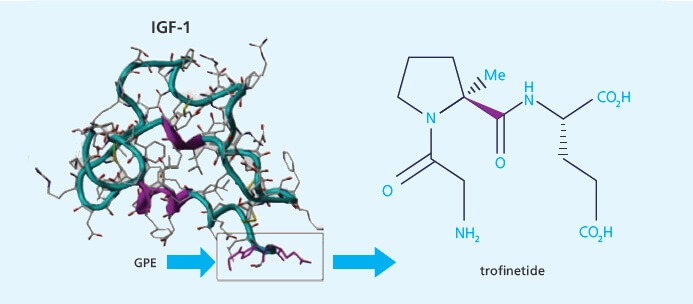
Trofinetide Clinical Trial Results Published
New Zealand-Based Biotech Neuren Pharmaceuticals Has Published Successful Phase 2 Fragile X Clinical Trial. Trofinetide, given to adolescent and adult males with Fragile X syndrome, was shown to be generally safe and was well-tolerated. It also showed preliminary evidence of efficacy. This trial validated a new design which can be used in future trials.
Healx Raises $56M to use AI to Find Treatments for Fragile X & Other Rare Diseases
Healx has secured $56M in new financing to build a clinical-stage portfolio for rare diseases, including treatments for Fragile X syndrome, and to launch a global Rare Treatment Accelerator program. Where the traditional drug discovery model takes more than a decade and can run into the billions of dollars, Healx’s AI-driven approach makes the process faster, more efficient and cost-effective.
Tetra Announces $40M to Advance BPN14770 for FXS and Alzheimer’s Disease
Tetra Discovery Partners has signed a multi-part deal that could bring it up to $160 million, plus royalties, from Shionogi & Co, Ltd, a Japanese major research-driven pharmaceutical company. Tetra currently is conducting an investigational Phase 2 study of BPN14770 in adults with Fragile X Syndrome, an indication for which BPN14770 has received Orphan Drug Designation from the US Food and Drug Administration. This clinical trial was made possible by early work with the FRAXA-DVI and over $200,000 from FRAXA.
Finding Fragile X Biomarkers – From Transcriptomics to Behavior in Patients
FRAXA funded a study using blood-based transcriptomics to find reliable Fragile X biomarkers. This unique approach links molecular data to behavior for future trials.