CRISPR – Does it hold promise for Treatment of Fragile X Syndrome?
There’s been a lot of press concerning a new biotechnology called CRISPR/Cas9, or simply CRISPR. This technology, which is based on the discovery of naturally-occurring bacterial defense mechanisms, has attracted an enormous amount of biotech investment. It has also excited the imaginations of scientists, clinicians, and rare disease advocates everywhere. How might CRISPR be applied to Fragile X syndrome? CRISPR offers the tantalizing possibility of “editing” genes very precisely, and it could (theoretically) excise the methylated trinucleotide repeat sequence from Fragile X cells, rendering them entirely normal.
FRAXADev – Developing BK Channel Openers for Fragile X Syndrome
A number of people have asked us about FRAXADev, a new project starting in France; this is a nonprofit initiative which seeks to develop a new kind of drug for Fragile X. The drugs they are interested in testing in Fragile X clinical trials were developed by Bristol-Myers Squibb many years ago, and are now off patent. This class of drugs opens a potassium channel in the membrane of neurons, which helps to decrease neuronal excitability.
Crossroads of Fragile X and Alzheimers Research
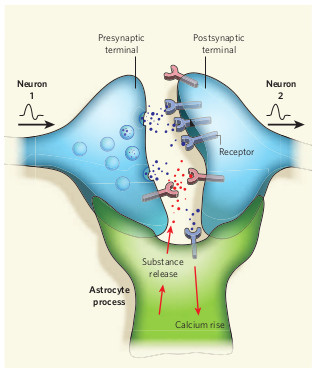
Last week researchers at VIB Leuven in Belgium published evidence that a brain pathway involving the protein APP (Amyloid Precursor Protein) plays a vital role in development of Fragile X syndrome, one of the most common causes of autism. Scientists led by Dr. Emanuela Pasciuto in the laboratory of Prof Claudia Bagni published findings of their study in the journal Neuron.
Fragile X: Past, Present, Future – Video
A Fragile X presentation was given by Michael Tranfaglia, MD, FRAXA Medical Director at the IDD-C conference at Stanford University on April 21, 2015. This was an in-depth discussion of how research has brought us to the point of clinical trials, the problems encountered in recent trials, and where we go from here. Dr. Tranfaglia presents new ideas on Fragile X disease mechanisms and new treatment strategies which may address these.
Fragile X Treatment: New Research Directions
In the wake of negative results from several high-profile clinical trials in Fragile X, we find ourselves questioning many of our previous assumptions about the nature of this disorder. After all, understanding the basic pathology of disease is critical to development of new treatments — this is true across the board, in all branches of medicine.
Bryostatin Restores Learning and Memory in Adult Fragile X Mice
A bizarre marine critter found off the California coast — Bugula neritina— is the only known source of a potential new Fragile X treatment, Bryostatin. Last month, FRAXA sat down with scientists from Neurotrope BioScience, a specialty biopharmaceutical company developing medicines for rare diseases and Alzheimer’s based on Bryostatin. Their Fragile X program is based on research by a West Virginia team led by Daniel Alkon, MD, which showed that Bryostatin-1 restores hippocampal synapses and spatial learning and memory in adult Fragile X mice.
Neuren’s NNZ-2566 Shows Clinical Benefit in Rett Syndrome Trial
This isn’t a Fragile X trial, but the Neuren compound, NNZ-2566, that is in trials now for Fragile X has shown significant positive effects in a Phase 2 trial for Rett syndrome. The results of the trial are interesting, in that improvement was seen a Rett syndrome-specific rating scale compared to placebo, and there was also improvement noted on the CGI-I (Clinical Global Impression of Improvement) and Caregiver Top 3 Concerns. However, there was no effect seen on ABC scores (Aberrant Behavior Checklist) compared to placebo. Many in the Fragile X field have noted the inadequacies of the ABC; indeed, it was never designed or intended to be an outcome measure for clinical trials.
NIH Awards $35 Million to Three Fragile X Research Teams
NIH is investing $35M in three Fragile X Research Centers. All teams have been funded by FRAXA and will now receive over $2M annually for five years.
Roche reports clinical trial negative results
Roche has shared the sad news that their clinical trials in Fragile X have been unsuccessful. They will host a Webcast on Thursday, September 18, from 12:30pm – 1:30pm (EDT) to explain the results. For details and dial-in information please see this letter from Luca Santarelli, the Head of Neuroscience, Ophthalmology and Rare Diseases at Roche Pharma Research and Early Development on…
Why Did Fragile X Clinical Trials of mGluR Antagonists Fail?
by Michael Tranfaglia, MD. In my opinion, the Fragile X clinical trials of AFQ056 sponsored by Novartis failed because of a dose range that was inadequate for Fragile X, and because of the unexpected development of tolerance.
Fragile X Clinical Trial: Novartis Trial Results Are In, and They’re Not Pretty
This year’s Gordon Conference just finished, and Novartis presented their results for the first time (though advisors and advocates had been given a private peak months ago.) To say that the trial results for AFQ056 were disappointing would be the understatement of the century!
Novartis Discontinues Development of mavoglurant (AFQ056) for Fragile X Syndrome
Mavoglurant trials in Fragile X did not show improvement vs. placebo, leading Novartis to end the program and wind down the open-label extension.
New Clue to Fragile X and Autism Found Inside Brain Cells
FRAXA-funded research revealed that mGluR5 isn’t only on the cell surface. Drugs may need to reach internal receptors to be effective in Fragile X.
Scientists Uncover Trigger for Fragile X Syndrome
A Weill Cornell team discovered that Fragile X stems from a gene being shut off—and a compound that blocks this process may prevent the condition.
Molecular mechanisms: Enzyme blockers help Fragile X mice
Dr. Jope won the 2013 FRAXA Pioneer Award for showing that lithium and other GSK-3–blocking drugs can reverse cognitive deficits in Fragile X mice.
Fragile X Syndrome Protein Linked to Breast Cancer Progression
Dr. Claudia Bagni’s team discovered that FMRP can act as a master switch in aggressive breast cancer, controlling proteins that drive invasion and metastasis.
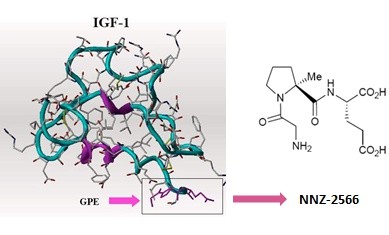
Fragile X Treatment Strategy Emerges from FRAXA Research: IGF-1
Neuren’s Trofinetide, part of a promising IGF-1 drug class, showed standout results in Fragile X mice—outperforming Rett syndrome models.
Lovastatin Discovery in Fragile X Mice Leads FRAXA to Fund Clinical Trials
FRAXA honored Dr. Emily Osterweil for discovering that lovastatin can correct key Fragile X abnormalities. Her findings were published in Neuron.
Phase 2b Clinical Trial of Arbaclofen in Autism Has Disappointing Results
The Phase 2b arbaclofen trial in autism didn’t improve social withdrawal, but did show progress on the Clinical Global Impression of Severity scale.
Clinical Trials FAQ ← Frequently Asked Questions
Wondering which Fragile X trial is right? Eligibility varies, so most families qualify for just one. Talk with your closest clinic to find the best fit.
FRAXA Progress and Future Plans
Huge momentum in Fragile X research: major results released, widespread media attention, and Phase III trials moving toward possible treatments.
What Works, and What Doesn’t
Early on, no one knew which path would work. Now the results are clear, and they’re directing FRAXA toward the next major Fragile X treatment breakthrough.
Compound that Inhibits mGluR5 Corrects Signs of Fragile X in Adult Mice
A Roche and MIT study published in Neuron finds that an mGlu5 inhibitor, CTEP, can reverse many Fragile X symptoms in adult mice.