Explore the latest breakthroughs in Fragile X research unveiled at the recent Banbury Meeting. Discover novel strategies, from gene therapy to protein replacement, that bring hope for curative therapies.
Read moreRett syndrome
Promising Results of Preclinical Study of ANAVEX®2-73

We are excited to share that Anavex Life Sciences announced today that preclinical data of the ANAVEX®2-73 (blarcamesine) study in Fragile X syndrome were published in the peer-reviewed journal, Scientific Reports.
Read moreUse of EEG as a Biomarker for Diagnosis and Outcomes in Neurodevelopmental Disorders

A series webinars focused on current topics in Fragile X research featuring Charles A. Nelson III, PhD, Professor at Harvard Medical School and Carol Wilkinson, MD, PhD, Instructor at Boston Children’s Hospital.
Read moreTrofinetide Clinical Trial Results Published
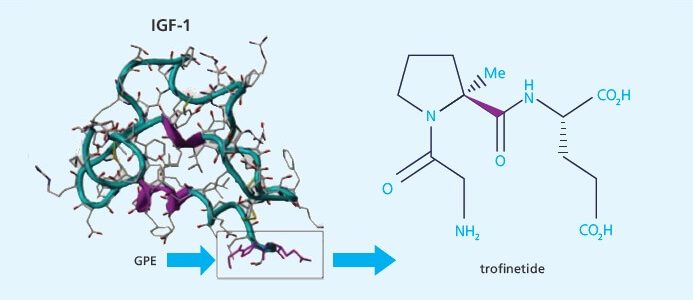
New Zealand-Based Biotech Neuren Pharmaceuticals Has Published Successful Phase 2 Fragile X Clinical Trial. Trofinetide, given to adolescent and adult males with Fragile X syndrome, was shown to be generally safe and was well-tolerated. It also showed preliminary evidence of efficacy. This trial validated a new design which can be used in future trials.
Read moreCan CRISPR Cure Fragile X Syndrome?

CRISPR/Cas9 was used by MIT researchers to remove the molecular tags that keep the mutant gene shut off in Fragile X syndrome neurons and resulted in some of them producing protein normally. Much work is being done right now, with exciting new discoveries coming at a fast and furious pace.
Read moreIn Their Own Words: Reports From the International Fragile X Workshop

The 18th International Fragile X and Related Neurodevelopmental Disorders Workshop in Quebec, Canada, was a great success, featuring Fragile X much more heavily than any previous meeting in this series! We asked our speakers to summarize their work in their own words, with brief updates from researchers investigating Fragile X.
Read moreIdentifying Biomarkers for Fragile X Syndrome – A Study in Argentina

Bio·mark·er, noun, a distinctive biological or biologically derived indicator of a process, event, or condition. Doesn’t help? Well, it’s perfectly clear to Argentinian researchers Patricia Cogram, PhD, and Paulina Carullo, MD, from the FLENI Institute in Buenos Aires, Argentina. They understand there is an urgent need for validated biomarkers after recent Fragile X syndrome clinical trials have failed on their primary endpoints.
Read moreAchieving Predictability: Developing Biomarkers for Fragile X Patients

New York University scientists make progress developing biomarker signatures and cataloging the types of Fragile X patients who will most likely benefit from new therapies. Take a closer look at your son or daughter with Fragile X syndrome. If you meet another child with Fragile X syndrome, chances are he/she may seem totally different to you, yet everyone is united under a FXS diagnosis. Discovering the biological reasons behind these differences is key to identifying which children will respond to what treatment. But how do you find the ‘prediction formula’? New York University scientists may soon know.
Read moreNew compound from Anavex Improves Learning and Behavior in Fragile X Mice
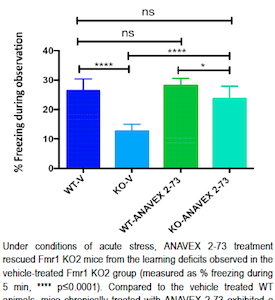
A potential new treatment for Fragile X syndrome is showing promise. While still early in development, the investigational drug was able to improve intellectual, learning and hyperactivity measures in a mouse model of Fragile X syndrome. Anavex 2-73 is a sigma-1 receptor agonist being developed for autism spectrum disorders, including Rett syndrome and Fragile X syndrome, and for Alzheimer’s disease. Anavex Life Sciences presented the data at the Gordon Research Conference for Fragile X and Autism-Related Disorders, held June 5-10, 2016 in Mount Snow, VT. The study was sponsored by FRAXA, via the FRAXA Drug Validation Initiative, and performed by Fraunhofer Chile Research, in Santiago, Chile.
Read moreThe X Factor – Turning on X Chromosome Genes to Treat X-linked Disorders

Harvard researcher Jeannie T. Lee, MD, PhD, moves closer to turning on select genes on the X chromosome to treat people with X-linked disorders.
Read moreNeuren’s Tofinetide Successful in Phase 2 Clinical Trial in Fragile X
We are pleased to share great news adapted from Neuren’s press release: Neuren’s phase 2 trial has successfully established proof of concept and provides a strong rationale for Neuren to move forward with developing trofinetide for Fragile X syndrome. In this initial small trial with a relatively short treatment period, trofinetide was very well tolerated, with the high dose (70 mg/kg twice daily) demonstrating a consistent pattern of clinical improvement, observed in both clinician and caregiver assessments.
Read moreNeuren’s NNZ-2566 Shows Clinical Benefit in Rett Syndrome Trial
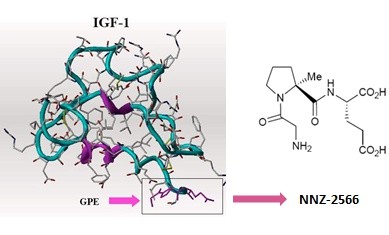
This isn’t a Fragile X trial, but the Neuren compound, NNZ-2566, that is in trials now for Fragile X has shown significant positive effects in a Phase 2 trial for Rett syndrome. The results of the trial are interesting, in that improvement was seen a Rett syndrome-specific rating scale compared to placebo, and there was also improvement noted on the CGI-I (Clinical Global Impression of Improvement) and Caregiver Top 3 Concerns. However, there was no effect seen on ABC scores (Aberrant Behavior Checklist) compared to placebo. Many in the Fragile X field have noted the inadequacies of the ABC; indeed, it was never designed or intended to be an outcome measure for clinical trials.
Read moreFragile X Treatment Strategy Emerges from FRAXA Research: IGF-1
New Zealand-based biotech Neuren Pharmaceuticals has announced impressive preclinical results in the Fragile X mouse model with Trofinetide. These compounds are examples of a new class of drugs based on insulin-like growth factors (IGF-1). IGF analogs are currently considered the most promising approach for treating Rett Syndrome, a fatal genetic disorder that affects only girls, and one of the other leading genetic models for the study of autism (along with Fragile X). The surprising news is that FRAXA researchers have found that this treatment strategy works even better in Fragile X knockout mice than in Rett syndrome mice! FRAXA’s strategy is to find and target the critical bottlenecks which block the way to development of treatments.
Read moreRole of Matrix Metalloproteinases (MMP-9) in Fragile X

With a $220,000 grant from FRAXA Research Foundation over 3 years, Dr. Iryna Ethell from the University of California at Riverside studied the regulation of dendritic structure by matrix metalloproteinases and other extracellular signaling pathways. This work identified a major treatment strategy for Fragile X with the available MMP-9 inhibitor, minocycline.
Read moreFMR1 Repression and the Signals to Chromatin

With a $70,000 grant from FRAXA Research Foundation from 2001-2003, Dr. Assam El-Osta and his team at the Peter MacCallum Cancer Institute studied mechanisms of methylation dependent silencing of FMR1, as well as regulation by histone acetylation/deacetylation.
Read more
