NIH Investigator Carolyn Beebe Smith, PhD, Looks to Improve Sleep in Fragile X Syndrome
Our sons with Fragile X Syndrome typically go to bed early and rise early. Sometimes they jump on us while we are sleeping at 3 a.m., excited to start their day. For heaven’s sake, why? The answer may come from Carolyn Beebe Smith, PhD, senior investigator, Section on Neuroadaptation and Protein Metabolism, National Institute of Mental Health, National Institutes of Health, Bethesda, Maryland. She is studying why children, in particularly boys, with FXS have problems sleeping.
New Fragile X Clinical Trial for Children Launching in June 2017
Rush University Medical Center Professor Elizabeth M. Berry-Kravis, MD, PhD, has launched and is recruiting participants for a large-scale clinical trial to study effects of AFQ056, an mGluR5 blocker, on learning in young children.
Mark Bear’s Goal: Disease-Modifying Treatments for Fragile X
Researcher Mark Bear, PhD, Picower Professor of Neuroscience, sees success developing disease-modifying treatments for Fragile X syndrome and other developmental brain disorders. Finally, hope. And it comes from his lab, The Picower Institute for Learning and Memory, Department of Brain and Cognitive Sciences, Massachusetts Institute of Technology.
Function of FMRP and Test of a Novel Therapeutic Approach in a Fragile X Mouse Model
FRAXA-supported work has identified DgkK as a critical enzyme lost in Fragile X. Drugs that raise DgkK levels may correct brain signaling and improve symptoms.
Sensory Hypersensibility in Fragile X Syndrome and BK Channel Openers
With $366,100 in FRAXA funding, researchers tested BK channel–opening drugs to fix sensory abnormalities in Fragile X mice; early results showed broad behavioral rescue.
Fragile X Mutant Mouse Models
With $375,000 in grants from FRAXA, Dr. David Nelson developed an array of advanced mouse models of Fragile X. These models are available at Jackson Labs (JAX).
MicroRNAs as Biomarkers in Fragile X Syndrome
The team at Johns Hopkins University studied groups of small RNAs, known as microRNAs, which are greatly decreased in brain tissue of Fragile X mice vs. normal controls.
Repurposing Drugs to Dampen Hyperactive Nonsense-Mediated Decay in Fragile X Syndrome
FRAXA-funded research showed nonsense-mediated mRNA decay is overactive in Fragile X, pointing to existing NMD-suppressing drugs like caffeine as potential treatments.
Kimberly Huber, PhD, Explores Hyperexcitability in Fragile X Syndrome
What causes hyperexcitability? Dr. Kimberly Huber seeks to understand how FMRP regulates connections between brain cells and the function of brain circuits.
University of Michigan researcher Peter Todd, MD, PhD, Aims to Selectively Turn the Fragile X Gene Back on in Human Cells
Fish like salmon are born in fresh water streams and rivers. When the time comes for them to breed, they return to the stream of their birth to lay eggs in the same spot where they were born. To accomplish this, they must swim upstream against the current or flow of the stream. Taking a page out of the salmon DNA playbook, University of Michigan scientists Peter Todd, MD, PhD, and postdoctoral fellow Jill Haenfler, Ph.D., are exploring unchartered waters to find a cure for Fragile X Syndrome. The researchers are adapting CRISPR research to reactivate the FMR1 gene, which provides instructions for making a protein called FMRP — needed for normal brain development.
Researcher David Nelson, PhD, Explores New Cell Strategies for Fragile X Syndrome, FXTAS and FXPOI
It’s rare to find a researcher working on the Big Three — Fragile X Syndrome (FXS), Fragile X-associated Tremor/Ataxia Syndrome (FXTAS) and Fragile X-associated primary ovarian insufficiency (FXPOI). Then again, David Nelson, PhD, is the rare bird. Nelson is a professor of Molecular and Human Genetics, Baylor College of Medicine, and director of Baylor’s Graduate Program in Integrative Molecular and Biomedical Sciences. He has been involved in FXS research since the late 1980s where he helped identify the mutation and the FMR1 gene. These days, researchers in Nelson’s lab at Baylor are studying FXS, FXTAS and FXPOI using mouse models.
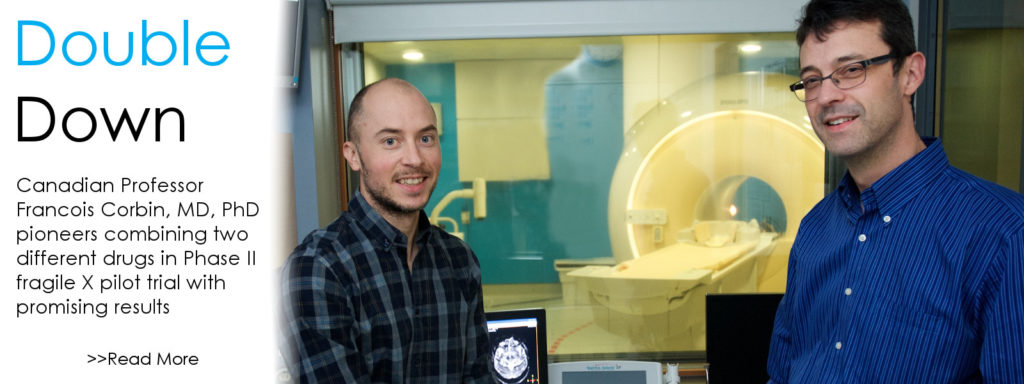
Double Down: Fragile X Clinical Trial Combines Two Available Drugs
If all the science world’s a stage, Fragile X researchers are more than merely players. They are center stage. So believes Francois Corbin, MD, PhD, professor, Université de Sherbrooke, Canada, who directs the university’s Fragile X Clinic. Corbin, who has received more than $100,000 in FRAXA support since 2012, is leading a pilot randomized Phase II trial, exploring the tolerability and the synergistic effect of a combined therapy.
The X Factor – Turning on X Chromosome Genes to Treat X-linked Disorders
Harvard researcher Jeannie T. Lee, MD, PhD, moves closer to turning on select genes on the X chromosome to treat people with X-linked disorders.
Fragile X Fruit Fly Research Bears Fruit
A new FRAXA-funded study shows how the hormone insulin – usually associated with diabetes — is involved in the daily activity patterns and learning deficits in the fruit fly model of Fragile X Syndrome (FXS). The study also reveal a metabolic pathway that can be targeted by new and already approved drugs to treat Fragile X patients, notably metformin.
Abnormalities of Synaptic Plasticity in the Fragile X Amygdala
With FRAXA funding, Dr. Sumantra Chattarji at NCBS explored how Fragile X alters amygdala function. Results were published.
Targeting AMP-Activated Protein Kinase Pathway in Fragile X Syndrome
With this grant from FRAXA, Dr. Peter Vanderklish explored AMPK activators to treat Fragile X. Both metformin and resveratrol, found in red wine, are AMPK activators.
Fruit Flies to Model and Test Fragile X Treatments
Boosting cAMP signaling restores memory and fixes brain-signaling defects in Fragile X models, suggesting diabetes drugs like metformin may help.
Analysis of Developmental Brain Dysfunction in Families
No strong behavioral similarities were found between parents and children with Fragile X, indicating family history may not guide clinical trial recruitment.
The Endocannabinoid System in a Mouse Model of Fragile X Syndrome
Fragile X disrupts endocannabinoid signaling. This study in mice demonstrated that correcting it may calm brain hyperexcitability and improve symptoms.
Inhibitors of STEP as a Novel Treatment of Fragile X Syndrome
STEP inhibition reversed behavioral and synaptic Fragile X deficits in mice (Neuropharmacology, 2018), highlighting STEP as a promising treatment target.
Molecular Mechanisms of Cytoskeletal Regulation by FMRP
With FRAXA funding, Dr. Jaffrey linked FMR1 loss to abnormal dendritic spines via RhoA signaling, suggesting RhoA-targeted therapies could help treat Fragile X.
Clinical Trials Outcome Measures
There is a critical need for reliable biomarkers and clinical outcome measures for Fragile X syndrome. Treatment trials depend on this.
Targeting the Endocannabinoid System in Adult Fragile X Mice
CB1 blockade with rimonabant reversed cognitive, sensory, and seizure symptoms in FXS mice, highlighting the endocannabinoid system as a therapeutic target.