Nospharma Announces Partnership with FRAXA to Test NOS-01 in Pre-Clinical Models of Fragile X
NOS-01 enters the FRAXA Drug Validation Initiative (FRAXA-DVI) for Fragile X mouse-model testing, generating data to guide development decisions.
Callum Cup IX Brings Community Together, Raising $20,000 for Fragile X Research
Callum Cup IX brought the Millburn FC community together, raising $20,000 for the FRAXA Research Foundation in support of Fragile X research.
FRAXA Drug Validation Initiative (FRAXA-DVI)
The FRAXA Drug Validation Initiative (FRAXA-DVI) provides speedy, cost-effective, objective preclinical testing to validate investigational and repurposed compounds for Fragile X.
Recruiting: Mirum Launches Clinical Trial of MRM-3379 for Fragile X Syndrome
Mirum’s MRM-3379 Phase 2 trial builds on FRAXA-funded PDE4D research and is now enrolling males ages 13–45 with Fragile X syndrome. View eligibility and study sites.
Shionogi Shares Update on Zatolmilast Fragile X Clinical Trials
Shionogi shares an update on its zatolmilast Fragile X clinical trials, outlining progress in U.S. studies and next steps in data analysis and evaluation.
Fragile X Research Q&A: Dr. Berry-Kravis on the RECONNECT Trial
Fragile X Research Q&A with Dr. Berry-Kravis on RECONNECT results, placebo effects, outcome measures, methylation, and research directions for families.
RECONNECT Trial Results: ZYN002 Does Not Meet Primary Endpoint in Fragile X
Harmony Biosciences announced that the Phase 3 RECONNECT trial of ZYN002 in Fragile X syndrome failed to meet its primary endpoint.
Fragile X Research Update: A Turning Point for Treatments and Curative Approaches
Fragile X research is at a turning point. FRAXA is funding ASO therapy and CRISPR-based gene reactivation to target the root cause of Fragile X.
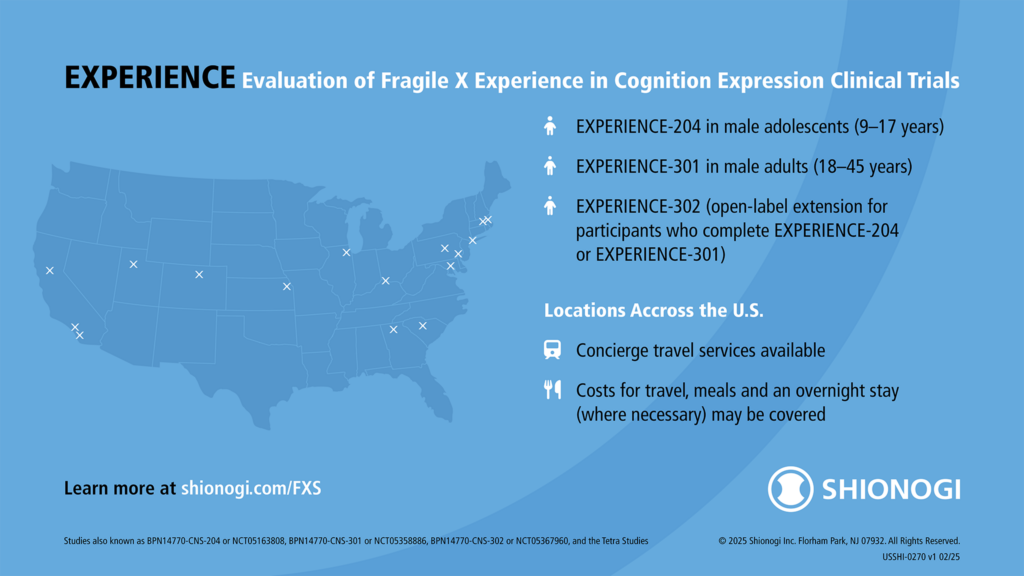
Shionogi’s EXPERIENCE Phase 3 Clinical Trial of Zatolmilast in Fragile X Syndrome
Learn more about Shionogi’s EXPERIENCE clinical trials for adults and adolescents with Fragile X syndrome, FRAXA’s role, and the open-label extension of these trials.
Callum Cup VIII Scores $19,400 for Fragile X Research – A Milestone Event
Callum Cup VIII, Millburn FC’s annual charity match, scored big with $19,400 raised for Fragile X research, bringing total funds to $148,000!
NPR Spotlights Zatolmilast: A Potential Breakthrough for Fragile X Syndrome
NPR spotlights zatolmilast, a promising investigational drug to treat Fragile X syndrome. Families report life-changing improvements in learning and independence.
Exploring Advances Fragile X Research: Comprehensive Webinar Highlights – May 2024
Learn about new FRAXA grants, key clinical trials, and scientific updates that are shaping the future of Fragile X syndrome. Webinar presented by Mike Tranfaglia and Katie Clapp.
Inside the FRAXA Drug Validation Initiative: Advancing Fragile X Treatments
FRAXA-DVI is revolutionizing Fragile X syndrome research, providing efficient, comprehensive and objective preclinical testing of potential treatments.
ASOs and Fragile X: Addressing the Most Asked Questions
Explore the potential of ASOs in treating Fragile X syndrome & FXTAS. Dive into a comprehensive Q&A addressing key questions and breakthrough findings.
Coming Together for Rare Disease Day 2023
Today we mark Rare Disease Day. FRAXA is committed to advancing research on Fragile X, one of the most common rare diseases worldwide.
Fragile X Clinical Trial of New PDE4D Inhibitor from Tetra
A $200K FRAXA grant enabled a successful Phase 2 trial of a PDE4D inhibitor for adult men with Fragile X, showing strong cognitive gains without side effects or tolerance.
Tetra’s Fragile X Clinical Trial – The Most Successful So Far
Dr. Mark Gurney, CEO of Tetra Therapeutics, discusses how one of the earliest clues to the biology of Fragile X led to the most successful Fragile X clinical trial to date. FRAXA and Tetra began working together after a key FRAXA-funded study caught the attention of Dr. Gurney. Through the FRAXA Drug Validation Initiative, Dr. Patricia Cogram was able to conduct preclinical validation experiments with Tetra’s lead compound in record time, paving the way for clinical trials.
Fragile X Syndrome: In Pursuit of a Cure Webinar
Global webinar “Fragile X Syndrome: In Pursuit of a Cure” on July 22, 2021 commemorated World Fragile X Day. Over 5,000 registered from more than 50 countries.
Tetra Releases Full Results of FRAXA-Funded Clinical Trial of PDE4D Inhibitor
Today, Tetra Therapeutics published the full results of its PDE4D trial published the full results to their announcement. Now having reviewed the full results, FRAXA can confidently say that the PDE4D drug trial gives hope to patients and families that Fragile X Syndrome is a treatable disorder, and this particular drug can improve intellectual disability.
Clinical Trials and Cyclic AMP in Fragile X Syndrome: A Life Journey
In November 2020, a phase II clinical trial reported extremely successful results. This clinical trial of a PDE4D inhibitor from Tetra Pharmaceuticals was conducted by Dr. Elizabeth Berry-Kravis at Rush University Medical Center and funded by FRAXA Research Foundation. In this Simons Foundation lecture, Elizabeth Berry-Kravis traces 30 years of Fragile X research, from identifying its cause, through finding dozens of treatment targets, through a series of disappointing clinical trials.
Positive Results Reported in Phase II Fragile X Clinical Trial of PDE4D Inhibitor Zatolmilast from Tetra Therapeutics
Today, Tetra Therapeutics announces the first unequivocally positive phase 2 clinical trial in Fragile X syndrome, press release below. The results do not depend on carving out a subset of patients or post hoc analysis.
Companies Move to Advance Potential Cognitive Treatment for Fragile X
Tetra Therapeutics and Shionogi announced plans to expand their partnership supporting BPN14770, a treatment candidate for disorders marked by cognitive and memory deficits, including Fragile X syndrome and Alzheimer’s disease. The agreement builds on an earlier collaboration between the two companies, and aims to further accelerate BPN14770’s development and potential marketing. It is currently in clinical testing in both Fragile X and Alzheimer’s patients.
Should You Participate in a Fragile X Clinical Trial?
A Fragile X clinical trial of a new PDE4D allosteric inhibitor from Tetra Therapeutics is nearly complete. Right now there are 3 remaining spots open to males 18-45 years of age with Fragile X syndrome. Dr. Elizabeth Berry-Kravis at the Rush University Medical Center in Chicago is leading this trial. The drug being studied has a unique mechanism of action that might improve cognitive and memory function.
Screening 2,320 FDA-Approved Drugs for Potential Treatment of Fragile X
FRAXA funded a screen of 2,320 FDA-approved compounds in the Fragile X fly model to identify hits that improve memory and social behavior for advanced testing.