BK Channel Openers: A New Drug for Fragile X Is Ready for Clinical Trials
A promising new BK channel opener, SPG601 from Spinogenix, is entering clinical trials for Fragile X syndrome. Learn about its potential to restore synaptic function and address core symptoms.
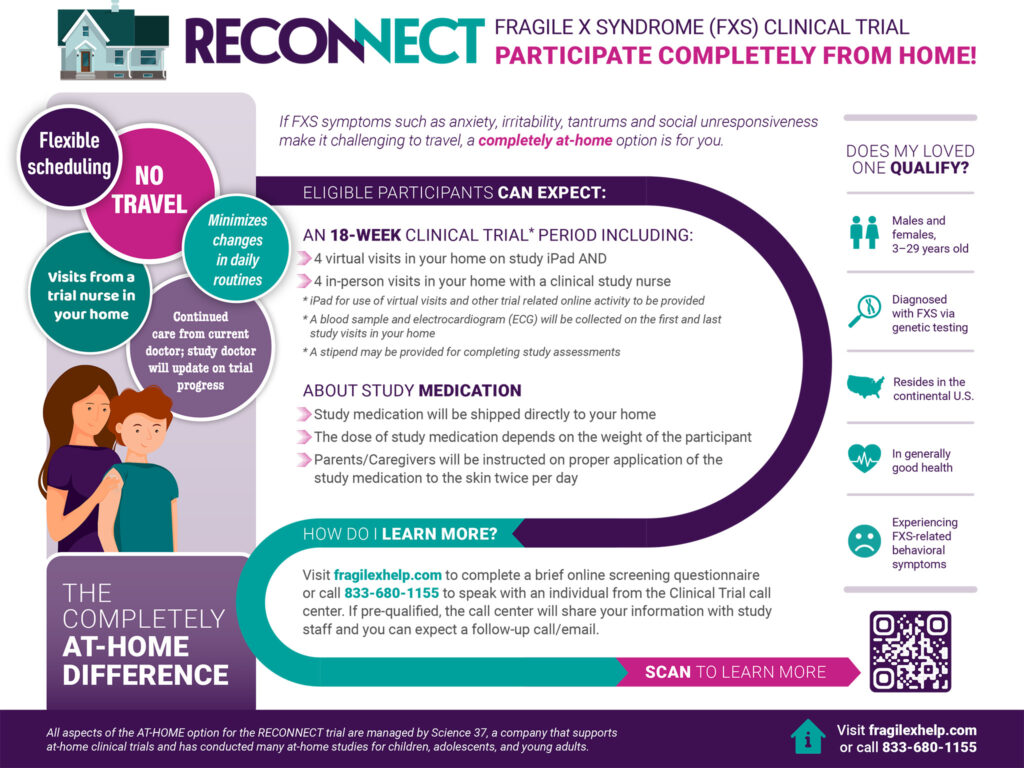
Harmony Biosciences Phase 3 Clinical Trial (RECONNECT) with At-Home Option
Harmony Biosciences’ RECONNECT Phase 3 trial of ZYN002 offered an at-home participation option. Unfortunately the trial reported negative results.
Renewed Hope: Navigating Towards a Cure for Fragile X Syndrome
Discover how Dr. Peter Todd’s latest Fragile X Syndrome research offers hope for advanced treatments and a possible cure, marking a new era in FXS therapy.
Inside the FRAXA Drug Validation Initiative: Advancing Fragile X Treatments
FRAXA-DVI is revolutionizing Fragile X syndrome research, providing efficient, comprehensive and objective preclinical testing of potential treatments.
Two-Med Combo Normalized Behavior, Improved Memory in Fragile X Mice
Treating Fragile X might require a combination of drugs. FRAXA-DVI tested ibudilast and gaboxadol in Fragile X mice. Together they rescued a wide array of symptoms.
An Update from Harmony Biosciences on Giving Tuesday
Harmony Biosciences completed its acquisition of Zynerba Pharmaceuticals. This message is from Jeffrey M. Dayno, M.D. Harmony’s President and CEO.
Virtual Research Q&A with Mike Tranfaglia, MD, and Katie Clapp
Please join FRAXA co-founders, Katie Clapp and Dr. Michael Tranfaglia, for a Research Q&A via Zoom on Wednesday, November 15, 2023 at 12:00 pm (noon) ET
FRAXA Research Foundation Partners with Autism BrainNet
FRAXA’s collaboration with Autism BrainNet can accelerate Fragile X syndrome research by collecting vital postmortem brain tissue.
ASOs and Fragile X: Addressing the Most Asked Questions
Explore the potential of ASOs in treating Fragile X syndrome & FXTAS. Dive into a comprehensive Q&A addressing key questions and breakthrough findings.
Pioneering Community-Based Drug Development for Fragile X Syndrome
Discover how FRAXA leverages Community-Based Drug Development to create impactful therapies for Fragile X syndrome. Join us as we reshape the future of rare disease treatment.
Innovative Breakthrough in Fragile X Treatment: The Promise of Antisense Oligonucleotide (ASO) Therapy
This changes everything! FRAXA funded research introduces Antisense Oligonucleotide (ASO) Therapy, redefining Fragile X syndrome treatment and understanding.
Unraveling Fragile X Syndrome: New Insights into FMR1 Gene Reactivation
Discover groundbreaking methods for reactivating the FMR1 gene in Fragile X syndrome. Dive into the transformational research and the implications of self-healing at a cellular level.
Allos Pharma Advances Phase 3 Clinical Trial Design for Potential Fragile X Syndrome Treatment, Arbaclofen
Discover Allos Pharma’s advancements in a pivotal Phase 3 trial for Fragile X syndrome treatment, Arbaclofen. Learn how their FDA-informed trial design might finally bring hope to the Fragile X community.
Breakthrough Discoveries in Fragile X Research: Insights from Special Banbury Meeting on Curative Therapies
Explore the latest breakthroughs in Fragile X research unveiled at the recent Banbury Meeting. Discover novel strategies, from gene therapy to protein replacement, that bring hope for curative therapies.
Fragile X Syndrome and Cancer Research: Unexpected Links and Opportunities for Collaboration
Discover unexpected links between Fragile X Syndrome and cancer. Studies show people with Fragile X have much lower cancer rates. Explore new opportunities for collaboration in this promising research.
FRAXA Investigator Lynne Maquat Awarded 2023 Gruber Genetics Prize
Dr. Maquat discovered NMD, a key surveillance system in the body that protects against mistakes in gene expression. With funding from FRAXA she is tackling Fragile X syndrome.
Sex-specific Learning Differences Found in Fragile X Patients, Mouse Model
Girls and women with Fragile X syndrome show different learning impairments relative to boys and men with the disease, a finding that was paralleled in a mouse model of the disease, a study found.
A Look Back at 2022 and Ahead to 2023 Research Prospects
FRAXA supporters met the $100K Giving Tuesday challenge—raising $269,744! Your actions truly spoke louder than words. Thank you!
10 Year Vision for Fragile X Research – Dr. Elizabeth Berry-Kravis & Dr. Patricia Cogram
In this video we hear from FRAXA Investigators Dr. Patricia Cogram, Professor at the University of Chile, and Dr. Elizabeth Berry-Kravis, Professor at Rush University Medical Center as they reflect on the progress that has been made and visualize what they see happening in the next 10 years for people living with Fragile X syndrome.
Clinical Trial Results Reported for Phase 3 CONNECT-FX Study of Zygel™
Results have just been published from Zynerba Pharmaceuticals’s phase 3 clinical trial of Zygel™ in the Journal of Neurodevelopmental Disorders. In this trial, 212 children and adolescents aged 3 to 17 years were given Zygel or placebo for 12 weeks.
FRAXA Volunteer Participates in Peer Reviewed Medical Research Program for the Department of Defense
FRAXA advocate Jennifer Frobish served as a reviewer for the Department of Defense’s medical research program, evaluating Fragile X–related proposals.
Why Pharma Companies Take on Fragile X, Explained
Research aimed at finding Fragile X syndrome treatments is exploding. Why are so many pharmaceutical and biotech companies investing in this orphan indication? FRAXA chief scientific officer Dr. Michael Tranfaglia explains the many reasons Fragile X is such a hot topic.
FMR1 Renamed to Fragile X Messenger Ribonucleoprotein 1
The efforts of the European Fragile X Network (EFXN) have led to the renaming of the FMR1 gene to “Fragile X Messenger Ribonucleoprotein 1” gene and the Fragile X protein, FMRP, to "Fragile X Messenger Ribonucleoprotein." Families around the globe are celebrating the news as a significant step forward for acceptance and the removal of a term that evokes many negative feelings.
How FRAXA Prioritizes Research, Explained
Dr. Mike Tranfaglia explains how FRAXA prioritizes research and the importance of looking at research from multiple angles. "It’s not either-or. It’s not we have a definitive treatment or we have a new drug treatment or we have a repurposing treatment. We can have all of those things, mixed or matched, in a personalized medicine kind of way and I think that’s what we’re headed for."