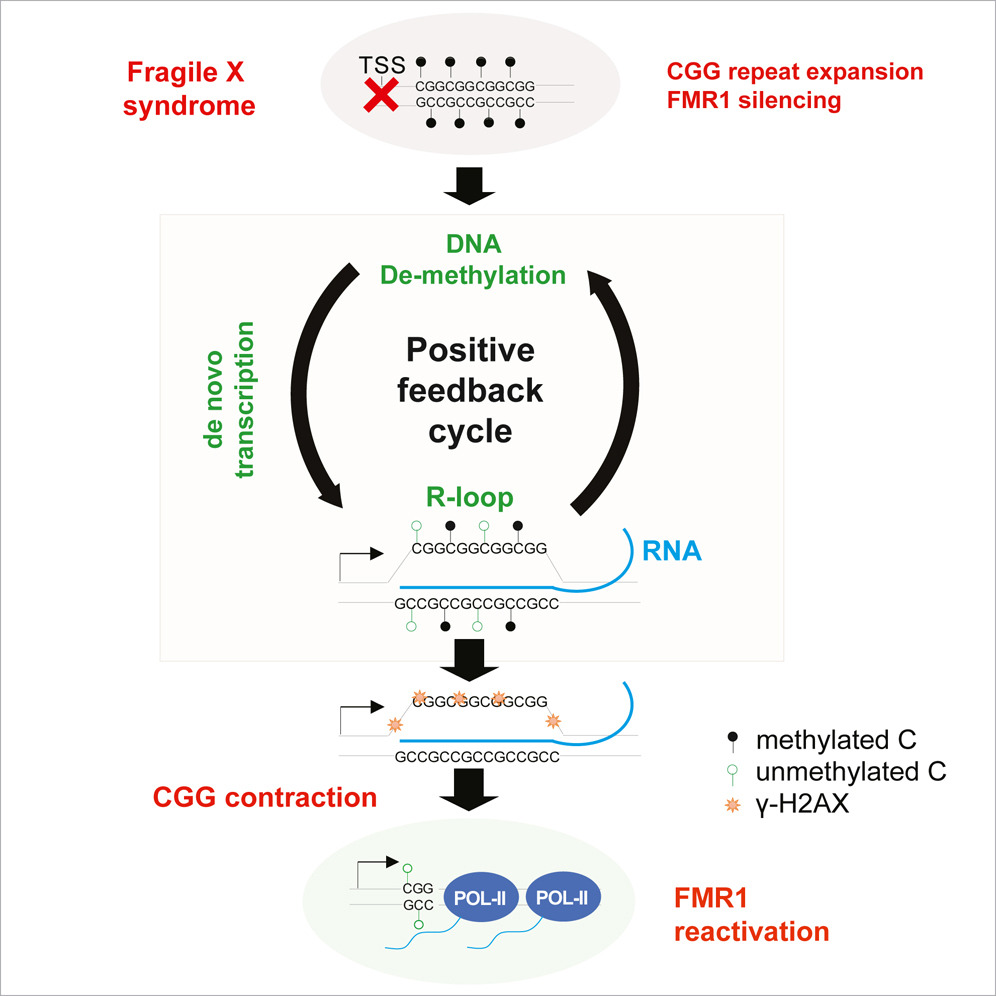
In a groundbreaking article titled "Site-specific R-loops induce CGG repeat contraction and fragile X gene reactivation", featured in the prestigious journal, Cell, Dr. Jeannie Lee and Dr. Hungoo Lee at Harvard Medical School and Massachusetts General Hospital present an ingenious method for reactivating FMR1, the gene silenced in Fragile X syndrome. FRAXA Research Foundation has funded this research for the past five years with the help of the Pierce Family Fragile X Foundation.
This research hypothesis sprang from an observation that cell culture lines originating from Fragile X embryonic stem cells sometimes revert to a pre-mutation state. Dr. Lee suggested that reversing cellular differentiation could potentially reactivate the gene and perhaps even reduce the trinucleotide repeat expansion.
A Closer Look at Stem Cells
Essentially, a stem cell is an unspecialized, less differentiated cell possessing the capability to grow into any other cell type. Certain cells can be transformed into stem cells, aka induced pluripotent stem cells, through exposure to multiple enzyme inhibitors. A widely recognized protocol employs a combination of five chemicals (called 5i) that inhibit five distinct enzymes. Remarkably, as per Dr. Lee's hypothesis, this protocol resulted in gene activation, protein re-expression, and over time, a reduction in repeat length.
Streamlining the Protocol for Fragile X Gene Reactivation
The next question was whether the effect on the FMR1 gene required all five chemicals, or if the protocol could be simplified. After rigorous experimentation, the researchers discovered that the process could be narrowed down to just two enzyme inhibitors. These two drugs alone could decrease the full mutation cells' repeat length to the pre-mutation range and reestablish normal protein expression levels within 12 days!
Another Method for Reactivating the Fragile X Gene
Dr. Lee's lab has also reported a second innovative method for achieving gene reactivation. Using a modified version of the CRISPR-Cas9 system that abolishes the Cas9 endonuclease activity ("dead" Cas9), they were able to reduce the repeat expansion, enabling the FMR1 gene to function normally. Interestingly, both the small molecule and dead Cas9 techniques seem to trigger the cell's natural DNA repair mechanisms. Other labs have attempted direct gene reactivation multiple times with limited success. However, Dr. Lee and colleagues have seemingly found two ways to induce cells to self-heal, which is more effective and likely more long-lasting.
Challenges and Caveats
It's important to note that these experiments were performed in dividing cells in a dish. Brain cells don't divide and are challenging to access, so this treatment strategy requires validation in non-dividing cells and whole brains. This presents a significant obstacle because the standard Fragile X mouse model is a knockout, meaning that the FMR1 gene is missing, so there is no trinucleotide repeat expansion to test. Surprisingly, even if researchers engineer a mouse with a very large repeat expansion added into its FMR1 gene, that mouse does not silence the gene (as humans do). Mice and humans really are not the same!
A Better Mouse
The next step is to test these techniques in rigorous in vivo models that closely emulate the human brain, while also assessing safety and the most efficient methods for delivering these treatments. While it has been challenging to model the trinucleotide repeat expansion in the mouse, there are potential ways to circumvent this technical limitation. For example, FRAXA addressed this problem years ago by funding research to implant human Fragile X cells into the brains of live mice. These cells are tagged with a dual-color dye system: one color identifies the cells which are human, and another color is produced only if FMRP is present. Researchers can now administer potential reactivation therapies to these live mice and search for Fragile X protein production in the human brain cells, which were otherwise non-producers.
Without a doubt, these discoveries will captivate the biotechnology industry's attention, leading to more investment and progress toward a cure!
Written by
Michael Tranfaglia, MD
Medical Director, Treasurer, Co-Founder
Dr. Michael Tranfaglia is Medical Director and Chief Scientific Officer of FRAXA Research Foundation, coordinating the Foundation’s research strategy and working with university and industry scientists to develop new therapeutic agents for Fragile X. He has a BA in Biology from Harvard University and an MD from the University of North Carolina at Chapel Hill. His son Andy has Fragile X syndrome.