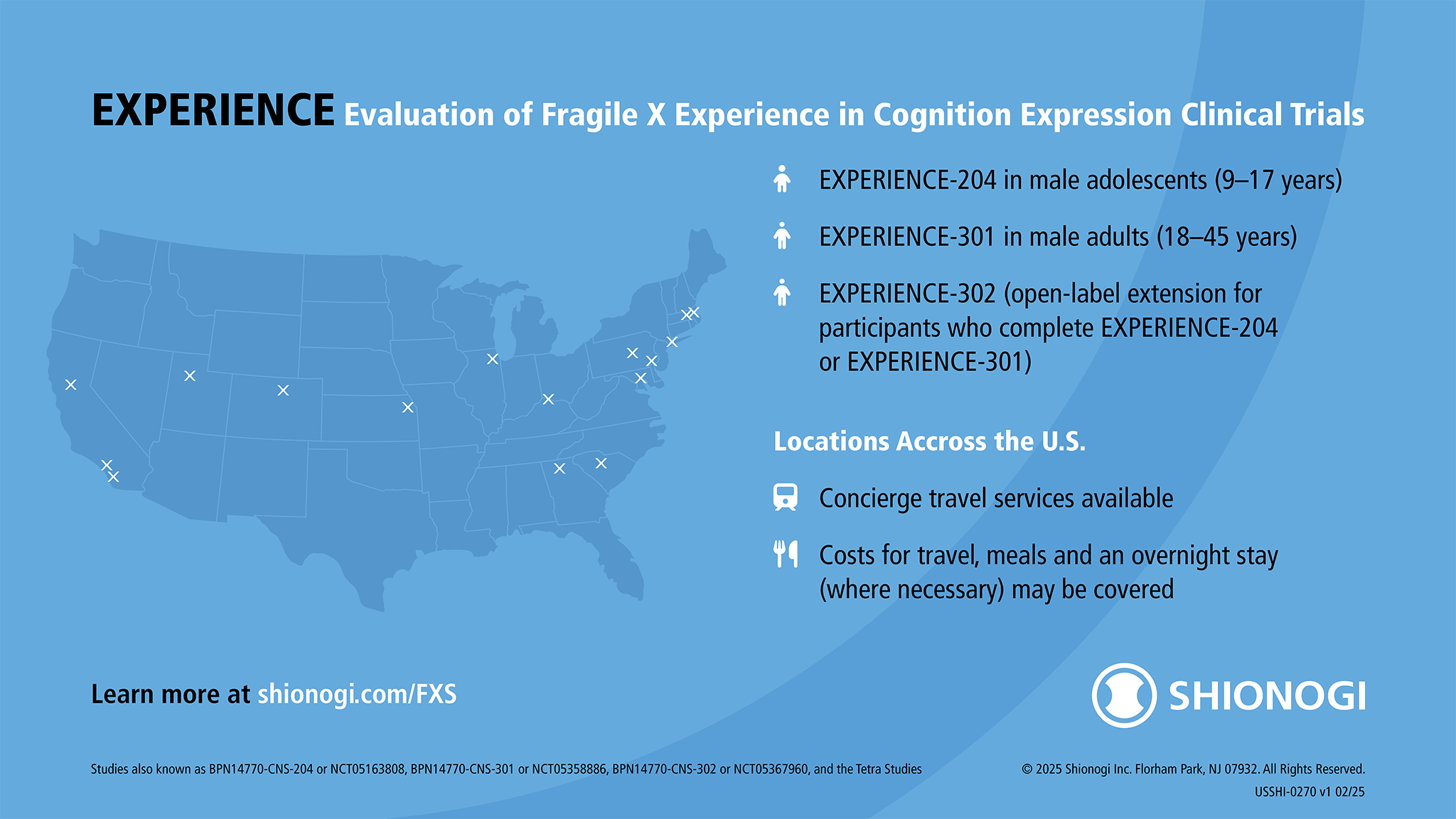
Shionogi’s EXPERIENCE Phase 3 Clinical Trial of Zatolmilast in Fragile X Syndrome
Updated 2025-08-05. Enrollment for these trials (EXPERIENCE-301 and EXPERIENCE-204 is complete. We are eagerly awaiting results.
Background on the EXPERIENCE Trials
The EXPERIENCE clinical studies, Shionogi’s ongoing Phase 2b/3 studies for an investigational drug for Fragile X syndrome, previously recruited qualified male participants aged 9–45 with a confirmed FMR1 gene mutation (≥ 200 CGG repetitions) at sites across the U.S.
You may have heard about EXPERIENCE (Evaluation of Fragile X Experience in Cognition Expression) as the Tetra studies or the studies of BPN14770. These studies are now being managed by Shionogi, who acquired Tetra Therapeutics in 2020.
Travel for study participants and their caregivers may be covered and can include transportation, lodging, and meal reimbursement (limitations may apply).
Find out about EXPERIENCE at www.shionogi.com/FXS. You can also contact Shionogi directly at medinfo@shionogi.com or 1 (800) 849-9707.

FRAXA's Role in Supporting This Research
These trials mark a major milestone for community-based drug development. FRAXA-funded research pointed the way to phosphodiesterase inhibitors to treat Fragile X many years ago. However, finding a good phosphodiesterase inhibitor was a challenge because there was so much interest in the pharmaceutical world in developing these drugs for Alzheimer's disease and other blockbuster indications.
FRAXA then partnered with Tetra Therapeutics to test their advanced PDE 4D inhibitor in Fragile X mice, with remarkable results.
The results in the mice justified clinical trials, so FRAXA and Tetra organized and co-funded the first Fragile X trials of this investigational drug. The outcome was a genuine breakthrough for the field: statistically significant improvement in Fragile X subjects on a wide range of outcome measures. Behavior improved, quality of life improved, electrophysiology improved, and most importantly of all, cognition improved markedly. There are no drugs currently approved to treat intellectual disability.
In 2020, Tetra Therapeutics became a wholly owned subsidiary of Shionogi & Co., Ltd., enabling the continuation and expansion of this research under Shionogi’s leadership.
If the new trials are successful, our community could see the first approved treatment for Fragile X syndrome on the market.
Post Revisions
- 2025/08 - Updated to report recruiting is complete.
- 2025/02 - Updated with additional recruiting sites and EXPERIENCE branding.
- 2025/01 - Updated to reflect additional recruiting sites and the transition of Tetra Therapeutics to Shionogi.
- 2024/02 - Updated to include new age range, protocol amendments and additional recruiting sites.
- 2023/10 - Updated to include open label extension trial.
- 2023/06 - Updated with additional recruiting sites.
- 2022/10 - Updated as clinical trial now recruiting at first location.
- 2022/07 - Original post published.