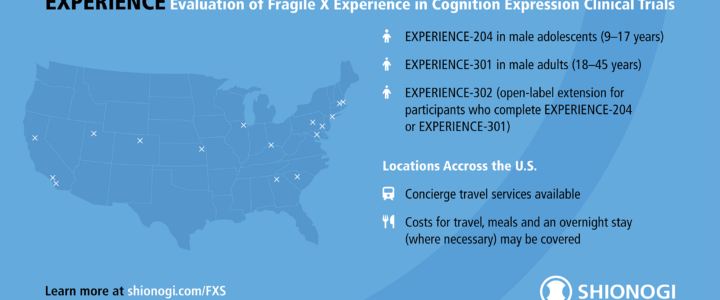
Shionogi’s EXPERIENCE clinical trials for Fragile X syndrome are nearing completion. Enrollment for the adult trial (EXPERIENCE-301) is now closed, while the adolescent trial (EXPERIENCE-204) is in its final phase. Learn more about the study, FRAXA’s role, and the open-label extension.
Read moreGurney, Mark
Positive Results Reported in Phase II Fragile X Clinical Trial of PDE4D Inhibitor Zatolmilast from Tetra Therapeutics

Today, Tetra Therapeutics announces the first unequivocally positive phase 2 clinical trial in Fragile X syndrome, press release below. The results do not depend on carving out a subset of patients or post hoc analysis.
Read more