Clinical Trials in Fragile X Syndrome
To all who have loved ones impacted by Fragile X syndrome, we sincerely thank you for your interest in clinical trials. These trials are beacons of hope during challenging times, and your curiosity and engagement are instrumental in pushing this important research forward.
As a further resource, we offer an enlightening video discussion featuring Holly Roos and Katie Clapp, both mothers to young adults with Fragile X and key figures in FRAXA. Their personal experiences provide unique and heartfelt perspectives on the journey through Fragile X clinical trials.
We understand that you might have questions about what participating in a clinical trial entails. Each study is supervised by a medical professional specializing in Fragile X syndrome. Participation is free, with no need to inform your insurance provider and the freedom to withdraw at any time. Typically, travel expenses are covered.
It's important to know that the FDA usually mandates two successful large-scale clinical trials before considering the approval of a new treatment, however for rare conditions like Fragile X syndrome, one trial with strong results may be sufficient. Upon successful trials and subsequent FDA approval, new drugs become accessible to others diagnosed with Fragile X syndrome, hopefully improving the quality of life for many individuals and families.
Ongoing Fragile X Studies Accepting Participants
FRAXA Research Foundation Joins COMBINEDBrain Consortium for Fragile X Biomarker Research
Help accelerate research on Fragile X syndrome biomarkers by contributing samples to the COMBINEDBrain Consortium’s project. Contact Katie Clapp at FRAXA Research Foundation to learn how you can participate.
Read More »Recruiting: Unveiling Probiotic Potential in Fragile X Syndrome Clinical Trial
First of its kind in Serbia, this clinical trial explores probiotic intervention as a potential treatment avenue for Fragile X syndrome.
Read More »Recruiting: BRIDGE Study (BRain Indicators of Developmental Growth)
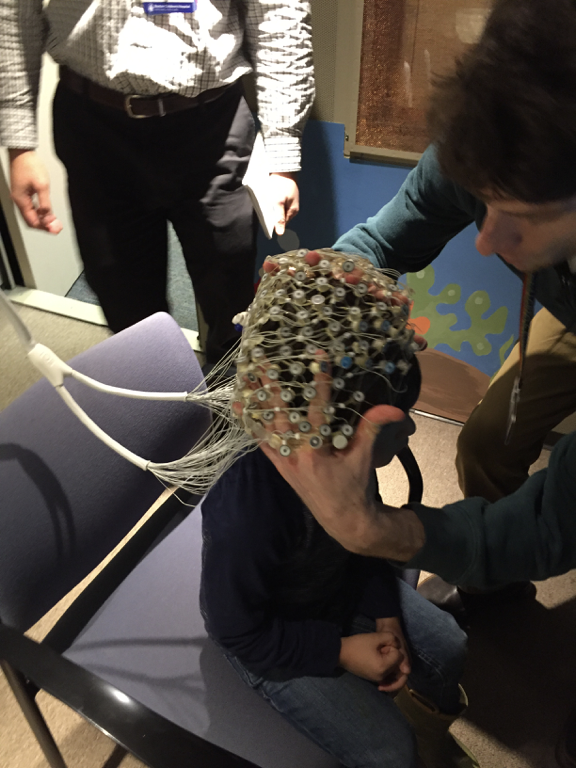
This study from the Wilkinson Lab at Boston Children’s Hospital is investigating how differences in brain activity affect learning, language and behavior in children with Fragile X syndrome, Down syndrome, and Autism Spectrum Disorder. One of the goals is to find brain markers that predict cognitive, language, and behavioral difficulties in these groups. Another goal is to better understand the differences in brain activity between young children with and without Fragile X and Down Syndrome, and whether these differences are similar in children with Autism Spectrum Disorder.
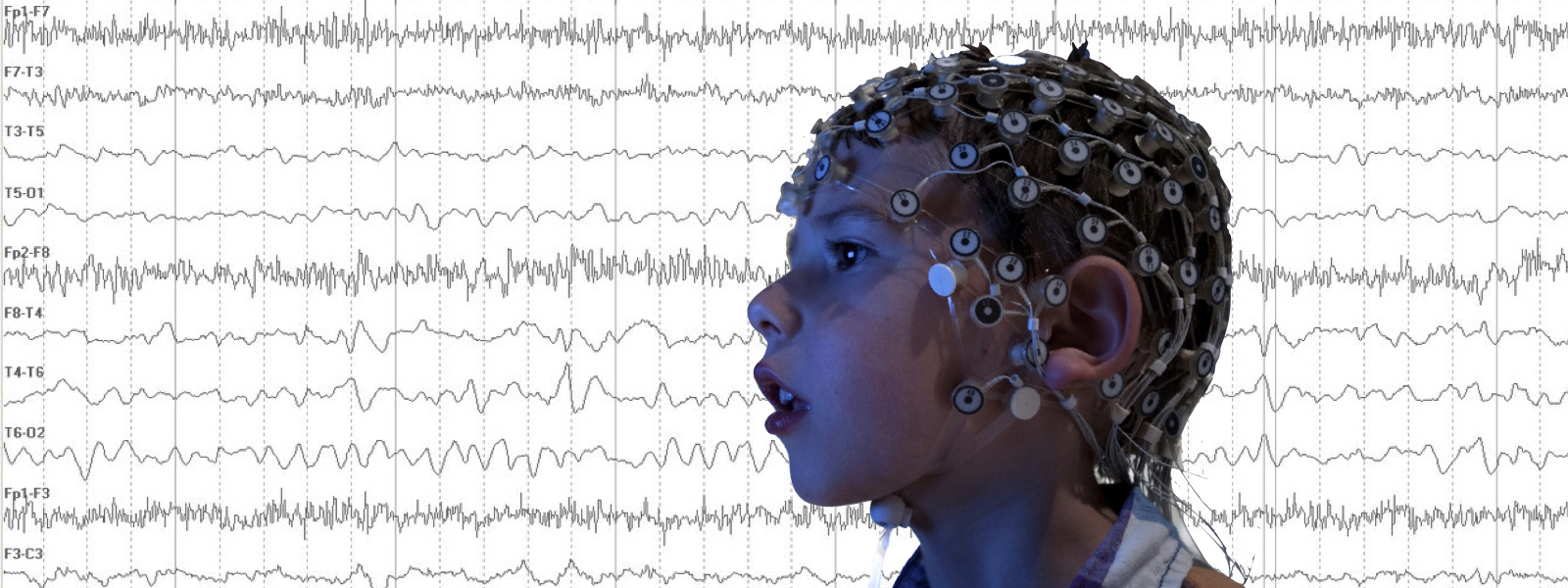
Read More »Recruiting: Clinical Study of Non-Invasive EEG for Children Ages 2-7
Dr. Carol Wilkinson, MD PhD, and Dr. Charles Nelson, PhD, at Boston Children’s Hospital are recruiting children ages 2-7 years with Fragile X syndrome to participate in a study of brain differences using non-invasive EEG.
Read More »Additional Fragile X Clinical Trials
Please visit clinicaltrials.gov for a complete listing of Fragile X syndrome clinical trials.