The FRAXA Drug Validation Initiative (FRAXA-DVI) provides speedy, cost-effective, objective preclinical testing of potential Fragile X treatments. FRAXA-DVI uses in-vitro systems, behavior batteries, and gene expression and peripheral biomarker platforms to validate investigational new drugs and repurposed available compounds in Fragile X syndrome (FXS).
Read moretrofinetide
Trofinetide Clinical Trial Results Published
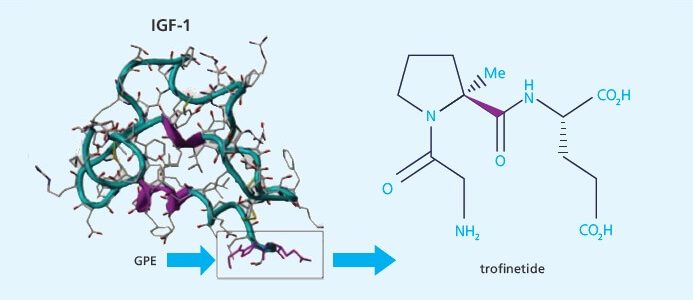
New Zealand-Based Biotech Neuren Pharmaceuticals Has Published Successful Phase 2 Fragile X Clinical Trial. Trofinetide, given to adolescent and adult males with Fragile X syndrome, was shown to be generally safe and was well-tolerated. It also showed preliminary evidence of efficacy. This trial validated a new design which can be used in future trials.
Read moreNeuren’s Tofinetide Successful in Phase 2 Clinical Trial in Fragile X
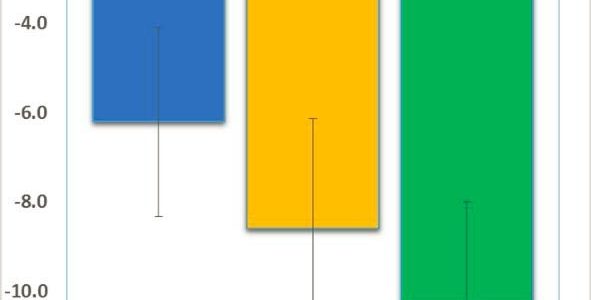
We are pleased to share great news adapted from Neuren’s press release: Neuren’s phase 2 trial has successfully established proof of concept and provides a strong rationale for Neuren to move forward with developing trofinetide for Fragile X syndrome. In this initial small trial with a relatively short treatment period, trofinetide was very well tolerated, with the high dose (70 mg/kg twice daily) demonstrating a consistent pattern of clinical improvement, observed in both clinician and caregiver assessments.
Read moreNeuren’s NNZ-2566 Shows Clinical Benefit in Rett Syndrome Trial
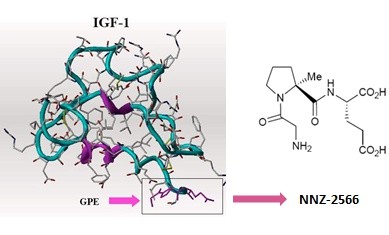
This isn’t a Fragile X trial, but the Neuren compound, NNZ-2566, that is in trials now for Fragile X has shown significant positive effects in a Phase 2 trial for Rett syndrome. The results of the trial are interesting, in that improvement was seen a Rett syndrome-specific rating scale compared to placebo, and there was also improvement noted on the CGI-I (Clinical Global Impression of Improvement) and Caregiver Top 3 Concerns. However, there was no effect seen on ABC scores (Aberrant Behavior Checklist) compared to placebo. Many in the Fragile X field have noted the inadequacies of the ABC; indeed, it was never designed or intended to be an outcome measure for clinical trials.
Read moreFragile X Treatment Strategy Emerges from FRAXA Research: IGF-1
New Zealand-based biotech Neuren Pharmaceuticals has announced impressive preclinical results in the Fragile X mouse model with Trofinetide. These compounds are examples of a new class of drugs based on insulin-like growth factors (IGF-1). IGF analogs are currently considered the most promising approach for treating Rett Syndrome, a fatal genetic disorder that affects only girls, and one of the other leading genetic models for the study of autism (along with Fragile X). The surprising news is that FRAXA researchers have found that this treatment strategy works even better in Fragile X knockout mice than in Rett syndrome mice! FRAXA’s strategy is to find and target the critical bottlenecks which block the way to development of treatments.
Read more
