Help accelerate research on Fragile X syndrome biomarkers by contributing samples to the COMBINEDBrain Consortium’s project. Contact Katie Clapp at FRAXA Research Foundation to learn how you can participate.
Read moreRecruiting
Recruiting: Unveiling Probiotic Potential in Fragile X Syndrome Clinical Trial

First of its kind in Serbia, this clinical trial explores probiotic intervention as a potential treatment avenue for Fragile X syndrome.
Read moreRecruiting: BRIDGE Study (BRain Indicators of Developmental Growth)
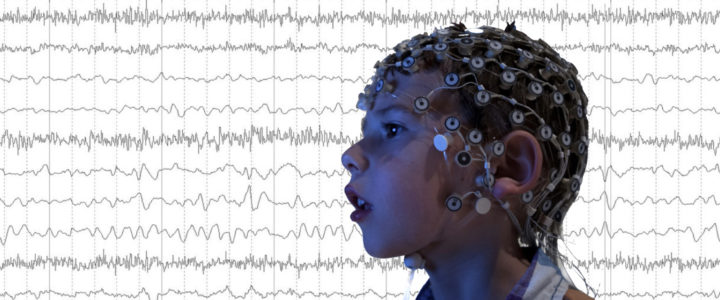
This study from the Wilkinson Lab at Boston Children’s Hospital is investigating how differences in brain activity affect learning, language and behavior in children with Fragile X syndrome, Down syndrome, and Autism Spectrum Disorder. One of the goals is to find brain markers that predict cognitive, language, and behavioral difficulties in these groups. Another goal is to better understand the differences in brain activity between young children with and without Fragile X and Down Syndrome, and whether these differences are similar in children with Autism Spectrum Disorder.
Read moreRecruiting: Clinical Study of Non-Invasive EEG for Children Ages 2-7

Dr. Carol Wilkinson, MD PhD, and Dr. Charles Nelson, PhD, at Boston Children’s Hospital are recruiting children ages 2-7 years with Fragile X syndrome to participate in a study of brain differences using non-invasive EEG.
Read more