Finding May Explain Many Brain Disorders, Lead to Prevention and Treatment

adapted from Weill Cornell Medical College press release
A new study led by Weill Cornell Medical College scientists shows that Fragile X syndrome occurs because of a mechanism that shuts off the gene associated with the disease. The findings, published today in Science, also show that a compound that blocks this silencing mechanism can prevent Fragile X syndrome – suggesting a similar therapy may be possible for 20 other diseases that range from intellectual disability to multisystem failure.
While researchers have known for more than two decades that the culprit behind Fragile X is an unusual mutation characterized by the excess repetition of a particular segment of the genetic code, they weren’t sure why the presence of a large number of these repetitions – 200 or more – sets the disease process in motion.
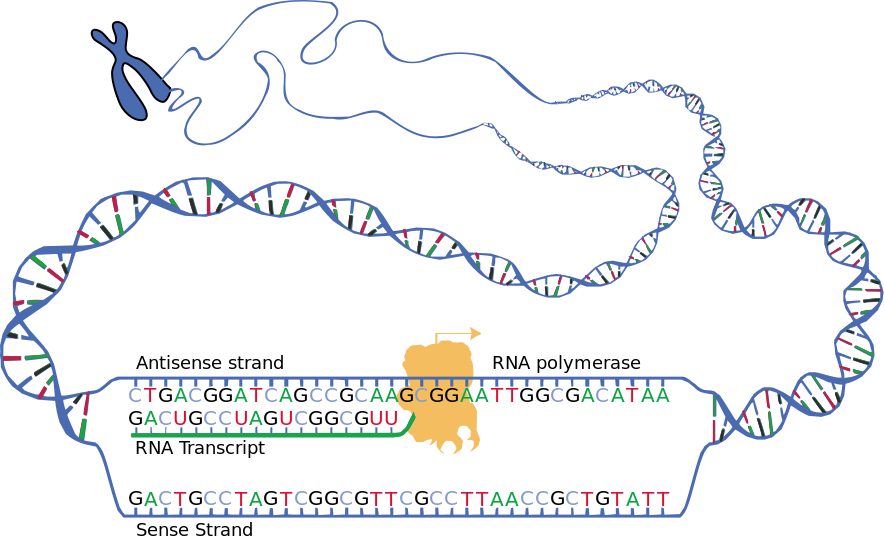
Using stem cells from donated human embryos that tested positive for Fragile X syndrome, the scientists discovered that early on in fetal development, messenger RNA — a template for protein production — begins sticking itself onto the Fragile X gene’s DNA. This binding appears to gum up the gene, making it inactive and unable to produce a protein crucial to the transmission of signals between brain cells.
“Until 11 weeks of gestation, the Fragile X syndrome gene is active – it produces its messenger RNA and protein normally. Then, all of a sudden it turns off, and stays off for the rest of the patient’s lifetime, causing Fragile X syndrome. But scientists have not understood why this gene gets shut off,” says senior author Dr. Samie Jaffrey, a professor of pharmacology at Weill Cornell Medical College and former FRAXA grantee. “We discovered that the messenger RNA can jam up one strand of the gene’s DNA, shutting down the gene — which was not known before.
“This is new biology — an interaction between the RNA and the DNA of the Fragile X syndrome gene causes disease,” Dr. Jaffrey says. “We are coming to understand that RNAs are powerful molecules that can regulate gene expression, but this mechanism is completely novel — and very exciting.”
The malfunction occurs suddenly — before the end of the first trimester in humans and after 50 days in laboratory embryonic stem cells. At that point, the messenger RNA produced by the Fragile X gene makes what the researchers call an RNA-DNA duplex — a particular arrangement of molecules in which the messenger RNA is stuck onto its DNA complement.
The RNA-DNA duplex then shuts down production of the Fragile X syndrome gene, causing the loss of a protein needed for communication between brain cells. The gene then remains inactive for life.
A normal Fragile X gene — one with fewer than 200 CGG repeats — stays active in a person without the disorder, and produces the necessary protein. However, the mutant Fragile X gene contains more than 200 CGG repeats, resulting in Fragile X syndrome.
“Because the Fragile X syndrome mutation is a repeat sequence, it is very easy for just a small portion of this sequence in the messenger RNA to find a matching repeat sequence on the DNA,” Dr. Jaffrey says. “This is a unique feature of repeat sequences. When there are 200 or more repeats, the RNA-DNA interaction locks into place.”
Hope for Treatment – and Other Disorders

Dr. Jaffrey and colleagues from The Scripps Research Institute in Florida and Albert Einstein College of Medicine in the Bronx, sought to find out why the disease is switched on when the CGG repeat is present in 200 to as many as 1,000 copies.
“Utilizing traditional ways to solve this puzzle has been impossible,” he says. “Human Fragile X syndrome genes introduced into mice and cells in the laboratory never turn off, no matter how many CGG repeats the genes have.”
So the scientists turned to human embryonic stem cells. They generated stem cell lines from donated embryos that tested positive for Fragile X syndrome. The stem cells were coaxed to become brain neurons, and at about 50 days, they differentiated in the same way that an embryo’s brain is developing at 11-plus weeks when the Fragile X syndrome gene is switched off.
The researchers then used a compound developed by FRAXA grantee Dr. Matthew Disney of the Scripps Research Institute that binds to CGG in the Fragile X gene’s RNA before and after the 50-day switch. Strikingly, the gene never stopped producing its beneficial protein.
That suggests a potential prevention or treatment strategy for Fragile X syndrome, Dr. Jaffrey says. “If a pregnant woman is told that her fetus carries the genetic mutation causing Fragile X syndrome, we could potentially intervene and give the drug during gestation. This may delay or prevent the silencing of the Fragile X gene, which could potentially significantly improve the outcome of these patients,” he says.
“This is a finding of enormous scientific interest, since it shows how direct, physical interaction between a gene and it mRNA product can lead to gene silencing,” said Michael Tranfaglia, MD, Medical Director of FRAXA Research Foundation. “It offers hope for a way to prevent Fragile X syndrome. However, a few caveats are in order. The compound tested in this study is not yet a drug; it is not ready to be tested as a drug, and it would take a long time to get it to that stage. It also can’t be tested in currently available Fragile X animal models, because we don’t have a Fragile X animal model with a silenced gene and with a trinucleotide repeat — the knockout mouse simply has a section of the gene removed. This strategy would also only work as prevention, not treatment of anyone with Fragile X. It is conceivable that there is some maintenance process ongoing in life, which could be blocked by this kind of drug, leading to gradual, long-term reversal of gene silencing — but this is entirely speculative and not addressed by this study.”
The researchers are now looking for similar RNA-DNA duplexes in other trinucleotide repeat diseases, including Huntington’s disease (a degenerative brain disease), myotonic dystrophy 1 and 2 (a multisystem progressive disease), Friedrich’s ataxia (a progressive nervous system disorder), Jacobsen syndrome (an intellectual disorder), and familial amyotrophic lateral sclerosis (a motor neuron disease), among others. “This completely new mechanism by which RNAs can direct gene silencing may be involved in a lot of other diseases,” Dr. Jaffrey says. “Our hope is that we can find drugs that interfere with this new type of disease process.”
This work was supported by the Tri-Institutional Stem Cell Initiative (Tri-I SCI) Grant 2008-019, New York Stem Cell Foundation-Druckenmiller Fellowship, Life Sciences Research Foundation Fellowship and Tri-I SCI postdoctoral fellowship, and a FRAXA postdoctoral fellowship. Portions of this project not involving non-NIH registry stem cells were supported by NIH R01 MH80420 and NIH R01 GM079235.

